-
在淡水和海洋生态系统中广泛存在的藻类是食物网基础[1],其光合作用占全球初级生产量近50%[2]。藻类种群形态多样性高[3-4],是最初作为藻类生物学分类的关键依据,后发现藻类形态与光和营养物质吸收存在一定关系[5]。MARGALEF[6]将细胞形态作为藻类季节演替的决定性因子,REYNOLDS[7]阐述了藻类形态与其生态竞争策略间的关系,藻细胞形态成为生态学研究的重要组成部分。物种形态学理论逐渐应用至藻类生态学,为解释和预测物种沿环境梯度的分布提供理论依据[8-9]。不同形态的藻类引发的水质问题不同,微囊藻的代谢产物微囊藻毒素会导致鱼类死亡同时危害人体健康,丝状藻的暴发会造成水华并产生水体嗅味问题[10]。而不同形态的藻类暴发时间同样存在差异,从藻类的形态及光合特性出发亦可解释藻类季节性分布规律。
不同环境条件被认为是影响藻类种内和种间形态多样性的最重要原因[11]。O’FARRELL[12]分析了水下光场对浮游植物的影响,发现在营养饱和条件下,相对较小的单细胞、无鞭毛生物、细丝状生物在光照有限的环境中具有优势,而有鞭毛和较大的生物在光照充足的环境中占优势。NASELLI等[13]观察到Arancio水库中水下有效光照与水库中藻类的主要形态之间存在显著相关性。SU等[14]利用光驱动下藻细胞形态演替模型阐释藻类群落的季节性演替规律。近年来,浮游植物形态特征和功能间的关系得到广泛研究,多项研究表明细胞形态与光环境条件变化有关[15-16]。如富含红色光的水体表面通常由球形形态和较短浮游植物细胞主导,而在总体光照强度较低且富含绿色光的水体深处,细长的杆状细胞和较长的浮游植物细胞分布更多[17]。MONTGOMERY[18]认为在自然环境中较低光强下细胞形态多样性更高,细长杆状细胞多,低光强环境下细胞体积改变引起细胞总光合膜表面积的变化可调节细胞的光合能力以更好适应环境光。SINGH等[19]综述了蓝藻中光信号对细胞形态的影响和对生物生理的潜在影响。
叶绿素荧光是藻细胞光合特性的关键指标,已成为藻类光合作用研究的热点。GENTY等[20]提出可利用基本荧光值来描述植物中光系统Ⅱ (PSII) 所吸收的激发能的分配,BILGER等[21]和WALTERS等[22]进一步研究提出了光合作用参数演算的方程:Y(II)+Y(NO)+Y(NPQ) = 1。其中,Y(II)是衡量照光状态下激发能中用于光化学反应的能量;Y(NO)是PSII处非调节性能量耗散的量子产量,是光损伤的重要指标;Y(NPQ)是PSII处调节性能量耗散的量子产量,为光保护的重要指标。相关研究多集中于区域内藻类整体情况,如李大命等[23]研究太湖蓝藻光合作用活性的时空间分布发现水体表面藻类Y(NPQ)与光强日变化一致,KASHINO等[24]发现巴芬湾水体藻类光合作用策略发现水体表层藻类Y(NPQ)更高。然而,藻细胞形态特征与光合特性间的相关关系研究仍不足,需要进一步确认两者如何共同影响藻类种群的时空分布。
为探明藻类的形态及光合特性如何影响藻类种群的时空分布,本课题组根据藻细胞形态特征拟选择45个藻种开展实验室研究,系统测定不同形态和光合特征参数,分析藻细胞形态与光合特征间的相关关系;结合于桥水库藻种监测数据,分析藻细胞光合特征时空变化规律,探讨基于藻细胞形态及光合特征的藻类种群时空分布机制,以期为湖库水体中有害藻类种群预警防控提供参考。
-
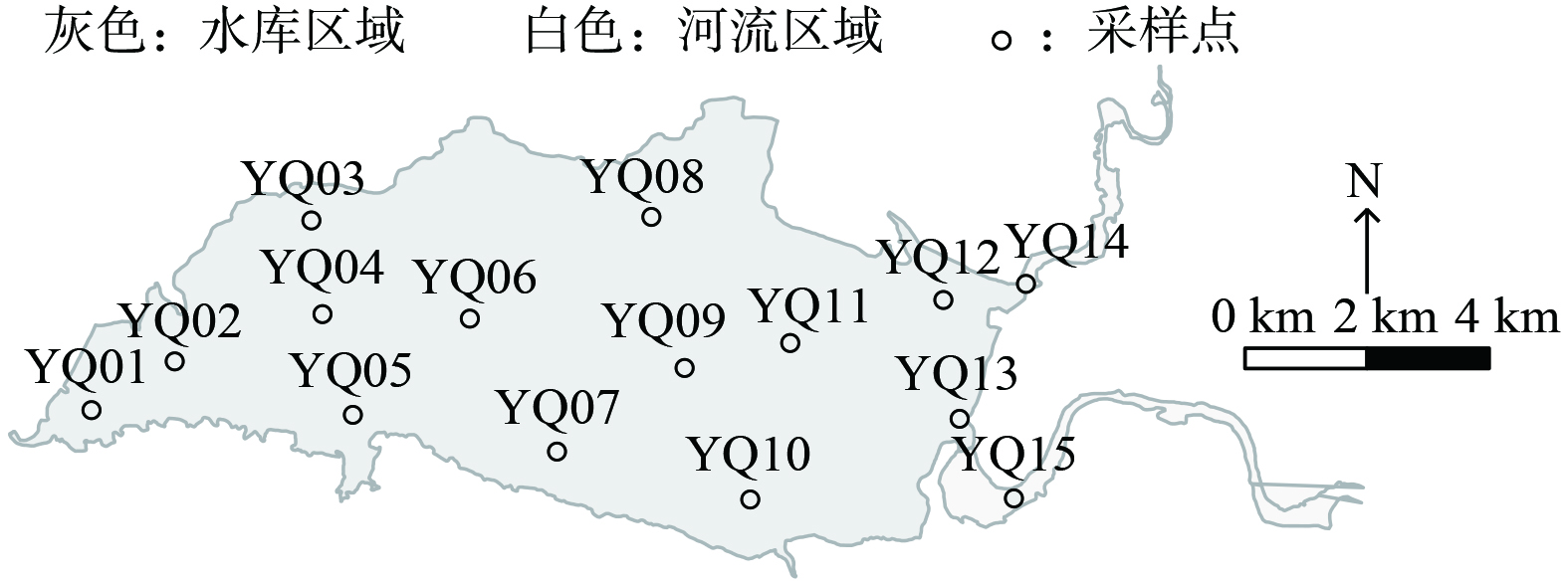
于桥水库 (40°00’~40°04’N,117°26’~117°37’E) 位于天津市蓟州区城东4 km处,流域面积2 060 km2,占整个州河流域面积的96%。最大回水长东西约30 km,南北宽8 km,最大淹没面积250 km2,正常蓄水位21.16 m时淹没面积86.8 km2[25],汛限水位19.87 m,死水位15 m。浅水库平均水深4.6 m,总库容15.59×108 m3。于桥水库是一座典型的山谷与平原过渡的水库[26],库区南岸地势陡峭受原河道深泓控制水深大,北岸地势则相对平坦水深浅[27]。由沙河、淋河、黎河三大支流汇合而成,其中黎河为引滦输水通道,与沙河汇流果河后随果河汇入于桥水库。
本研究在于桥水库布设的15个点位 (图1) ,分别于1月、4月、5月、6月、7月、8月、9月、10月、11月、12月进行浮游植物的采集 (2月、3月为冰封期,未进行采样) ,选择其中6个水库中不同位置的布设点分层采集浮游植物,在水库坝前 (YQ01)、库西 (YQ05)、库中 (YQ06)、库南 (YQ07)、库北 (YQ08)、库东 (YQ14) 水体浅水区表层 (0~2 m)、亚表层 (2~4 m)、中层 (4~6 m)、底层 (6~8 m) 采集水样。使用不同标定长度绳连接取水器原位取水1 L,装入聚丙烯瓶中保存,立即运至实验室进行预处理。于1%卢戈尔碘溶液中富集后保存在组织培养瓶 (25 cm2,康宁CLS3289,Sigma-Aldrich,美国) 中待后续镜检计数。
-
在24 h内使用光学显微镜 (Olympus BX51) 在200×、400×、600×和1 000×放大倍率下观察浮游植物样本,使用Sedgewick Rafter计数室进行计数协助鉴定。在本研究中,物种鉴定遵循PRESCOTT[28]、BELLINGER[29]、LING等[30]的方法,保存的子样本在沉淀室中静止至少48 h,然后浓缩10倍,最终在显微镜下进行细胞计数。
-
所使用藻种种类和培养基均购自中国科学院淡水藻种库 (表1) 。其中,蓝藻与绿藻采用实验室配置BG11培养基,所有藻类扩大培养后用于光合特性参数和形态参数测定。
-
根据藻细胞形态分类,构建了圆形、圆柱形、锥形、椭圆体形与长方体形共5种典型几何模型,使用研究级正置显微镜 (DM4B,德国) 测量藻类细胞的基本形态数据,通过SU等[14]的计算公式获得细胞投影面积 (CPA) ,最终得到同个藻种多个数据得算术平均值用于后续模型验证。
-
藻细胞的扁平化系数 (f) 表明了细胞形状偏离球形的程度,测得于桥水库的真光深度 (zeu) 和混光深度 (zmix) 。根据SU等[14]的计算方法可以得到,藻细胞的重叠系数为显微镜观察下纯藻藻细胞间的接触面积与自身表面积之比。
-
在样品在暗适应20 min后,使用浮游植物分类荧光仪PHYTO-PAM-II (德国) ,应用低强度测量光 (2 μmol·m−2 s−1,622 nm) 检测暗适应样品的固有荧光 (Fo) 。在200 ms多次翻转光化闪光期间达到稳定状态后,分别测量5种不同激发光下 (440、480、540、590、625 nm) 暗适应样品的最大荧光 (Fm),光适应样品的最大荧光 (
$F'_{\rm{m}} $ $F'_{\rm{o}} $ $F' $ $F' $ $F'_{\rm{m}} $ $F'_{\rm{o}} $ $F' $ $F'_{\rm{m}} $ $F'_{\rm{o}} $ -
在样品在暗适应20 min后,使用浮游植物分类荧光仪PHYTO-PAM-II (德国) ,应用0~2 000 μmol·m−2 s−1范围内20档测量光,光曲线的梯度设置依次递增,每一步照光的时间设置为20 s,分别测量每档光强下5种不同激发光 (440、480、540、590、625 nm) 的电子传递速率ETR,通过EP Mode[31]拟合光曲线得到光合特性参数α、ETRmax。
EP Mode拟合光曲线方程为式 (7) 。
式中:a,b,c 为可变参数。
光曲线的初始斜率α计算见式 (8) 。
最大电子传递速率ETRmax计算见式 (9) 。
半饱和光强lk计算见式 (10) 。
-
1) 藻类形态参数分布情况。本研究中45株藻种的细胞形态特征如表2所示,藻细胞投影面积 (CPA) 在 (2.61±0.67) μm2 (Leptolyngbya sp. 479) 和 (611±179) μm2 (Synedra ulna fa2597) 之间变化。大部分蓝藻CPA ( (12.0±14.1) μm2) 小于绿藻 ( (44.1±36.0) μm2) 和硅藻 ( (342±266) μm2) ,细胞比表面积 (SPSA) 呈现相反趋势,范围为 (0.0997±0.015 9) μm−1 (Peridinium umbonatum var. Inaequale fa329) 至 (3.93±0.33) μm−1 (Leptolyngbya sp. 479) ,蓝藻、绿藻和硅藻的SPSA平均值分别为 (2.44±0.92) μm−1、 (1.29±0.66) μm−1和 (0.733±0.181) μm−1。藻细胞扁平化系数 (f) 分布与门归属的相关性较低,绿藻的扁平化系数分布于 (0.1825±0.0032) 与 (0.6825±0.0246) 之间,蓝藻的扁平化系数为0~0.4。藻类重叠系数虽然与门归属相关性较低,但与藻类自身形状关系密切,重叠系数大于0的藻类多为蓝藻、绿藻中的丝状藻,而其他形状的藻类细胞重叠系数大多为0。
表2表明蓝藻的CPA小于绿藻和硅藻,而蓝藻的SPSA大于绿藻和硅藻,呈现出相反的分布规律。这可能与蓝藻是原核生物,而绿藻和硅藻是真核生物有关。PLOUG等[32]的研究发现,在较小的原核细胞中,较大的SPSA意味着营养物质可轻松快速地到达细胞内部的任何部分。相反,在较大的真核细胞中,有限的SPSA意味着营养物质不能迅速扩散到细胞的内部,真核细胞需要各种专门的内部细胞器来进行新陈代谢、提供能量,并在整个细胞内运输化学物质。
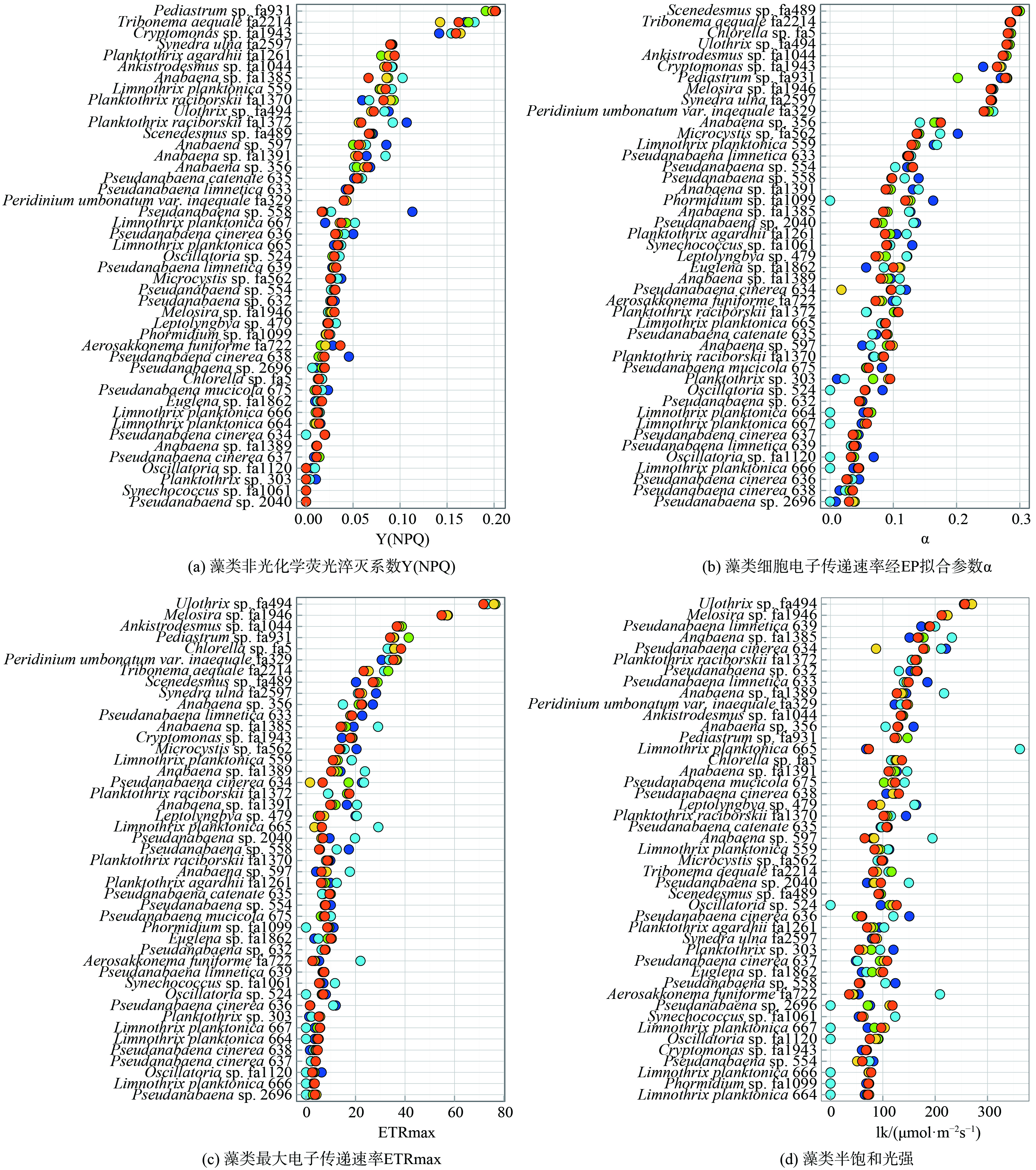
3) 藻类光合特性参数分布情况。同时研究了5种不同激发光下45株藻种的光合特性 (图2) ,不同藻种Y(NPQ)值主要分布在0 (Pseudanabaena sp. 2040)~0.207 (Pediastrum sp. fa931) ,大部分蓝藻Y(NPQ) (0.045±0.047) 小于绿藻 (0.126±0.099) 和硅藻 (0.071±0.027) (图2(a)) 。不同藻种α值分布在0 (Pseudanabaena sp. 2696)~0.3 (Scenedesmus sp. fa489) ,蓝藻α值 (0.082±0.083) 较小,绿藻 (0.280±0.078) 与硅藻 (0.270±0.016) 相对较大 (图2(b)) 。不同藻种的最大电子传递速率 (ETRmax) 在0 (Pseudanabaena sp. 2696)~76.5 (Ulothrix sp. fa494) ,并呈现出相同的趋势,蓝藻ETRmax值 (9.132±20.068) 小于绿藻 (43.33±33.27) 和硅藻 (39.72±18.72) (图2(c)) 。此外,各藻种的半饱和光强lk与藻种分类学特征无显著相关性,总体上分布在0 (Limnothrix planktonica 664)~361.8 (Limnothrix planktonica 665) (图2(d)) 。
图2(a)表明蓝藻的Y(NPQ) 普遍小于绿藻和硅藻,这可能是因为蓝藻相较于绿藻和硅藻拥有更小的CPA,捕光潜力低,在供给自身生长时无法兼顾光保护所需要的能量。此外,在图2 (b) 和图2 (c) 中还发现了一个有趣的现象,蓝藻在440 nm激发光条件下的ETRmax (10.24±16.76) 大于625 nm激发光条件下的ETRmax (7.75±16.65) ,而在440 nm激发光条件下的α (0.074±0.1) 小于625 nm激发光条件下的α (0.081±0.094) ,绿藻和硅藻中不存在此规律。MIAO等[33]研究了黄瓜叶片不同色光下能量分配的变化发现蓝光和红光对维持光合作用的电子传递同样重要,然而蓝光不被蓝藻中的藻胆蛋白吸收,蓝光条件下蓝藻的光系统Ⅰ与光系统Ⅱ能量不平衡,导致蓝藻对蓝光的利用效率低[34],在440 nm激发光条件下α值小。
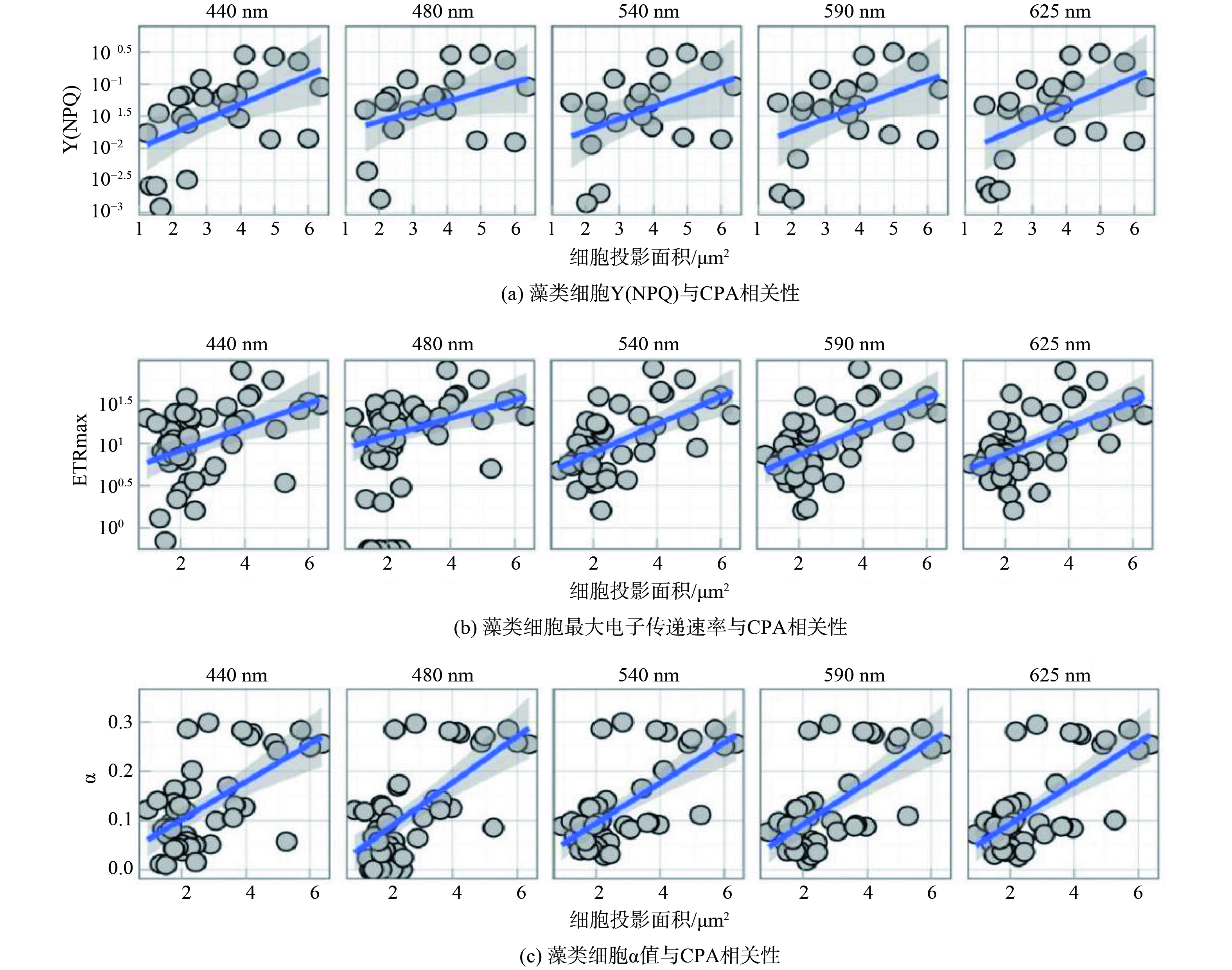
3) 藻类形态参数与光合特性参数相关性分析(图3)。基于藻种的形态参数CPA与光合特性参数Y(NPQ)、α、ETRmax构建线性模型,在5个不同波长激发光下,Y(NPQ)、α、ETRmax与CPA均呈正相关关系。Y(NPQ)与CPA的线性模型在五种激发光下斜率 (0.067±0.002) 与R2 (0. 31±0.001) 几乎相同,p值均小于0.001。在440 nm激发光条件下,ETRmax与CPA间的线性模型中斜率 (11.6) 最低,R2为 (0.25±0.05) ,p值均小于0.001。α与CPA间的线性模型中斜率 (0.107) 在480 nm激发光条件下时最大,R2为 (0.41±0.06),p值均小于0.001。
藻类形态在不同深度存在差异性,丝状蓝藻一般处于水体中底层[35],球状蓝藻多分布于表层水体[36]。不同形态藻类形态参数差异很大,已有对密云水库表层蓝藻和底栖蓝藻的演替过程的研究发现投影面积和光照强度能够影响蓝藻演替[37]。藻类的投影面积关系到其对光的接收能力,也能反映藻类的光收集潜力,基于不同藻种形态参数与光合特性参数构建线性模型发现藻类Y(NPQ)、α、ETRmax与CPA的正相关关系。藻类CPA越大,光接收能力越强,光合作用时的电子传递速率越快。对藻类电子传递速率进行EP拟合[31]所得到的光响应曲线斜率α反映了光能的利用效率。尽管蓝藻生活在水体亚表层和底层弱光环境内,但这并没有使得蓝藻对光的利用效率变高,反而是CPA较大的绿藻和硅藻拥有更高的光利用能力。研究发现藻类CPA与Y(NPQ)呈正相关关系,这表明更大的CPA为藻类提供了更强的光保护能力,可降低高光强对藻细胞的伤害,这也解释了蓝藻为何呈现形态分层的现象,球状蓝藻的CPA与Y(NPQ)远超丝状蓝藻,较强的光保护能力使得球状蓝藻能生存在水体表面。
-
图4 (a) 表明于桥水库藻类Y(NPQ)分布整体呈现出由春季到夏季藻类总体Y(NPQ)减小,从夏季到秋季藻类总体Y(NPQ)增大的趋势。冬季1月藻类Y(NPQ)为 (0.075±0.065) ,4月 (0.062±0.070) 与5月 (0.064±0.067) 藻类Y(NPQ)开始逐渐下降,在夏季6、7、8月藻类Y(NPQ) (0.058±0.073) 达到最低,9月藻类Y(NPQ) (0.064±0.067) 开始逐渐增加,经过10、11月藻类Y(NPQ) (0.067±0.065) 增加后,于12月藻类Y(NPQ) (0.071±0.061) 达到最高。图4 (b) 为于桥水库藻类Y(NPQ)时空分布情况,尽管不同季节藻类Y(NPQ)分布存在差异,但藻类Y(NPQ)均随着深度增加而降低,水体表层 (0~2 m) 的藻类Y(NPQ)一般分布于0.075左右,而水体亚表层 (2~4 m) 和水体底层 (4~8 m) 藻类Y(NPQ)相对较低分布于0.05左右。
图4 (a) 藻类Y(NPQ)的时间分布与SU等[38]发现的藻类季节性分布规律具有相似性,从春季到夏季藻类主导形态由饼状细胞转换为球状细胞,藻类Y(NPQ)随之降低;从秋季到冬季饼状细胞重新占据主导地位,藻类Y(NPQ)不断升高。图4 (b) 中不同季节下藻类Y(NPQ)在不同深度的分布阐明了Y(NPQ)在水体藻类形态空间分布中的重要性。在1月份由于低温对藻类生长的负影响使得藻种数量处于全年最低,限制了Y(NPQ)分布的差异性,4、5月份藻类开始复苏,与此同时藻种Y(NPQ)在深度上分布的差异性开始体现,温度上升和光照增强使得整体藻类Y(NPQ)降低。8、9月是藻类暴发风险最高的季节,藻种数量最多,藻类Y(NPQ)在不同深度的分布差异最大,呈现出了随着深度增加藻种Y(NPQ)递减的趋势。10、11月藻种数量开始减少,Y(NPQ)分布的差异性也降低。由于不同颜色的光波长不同,水体表层和底层光谱组成不同,水体表面富含波长较长的红光,水体深处波长较短的绿光和蓝光含量更高。BORDOWITZ等[16]研究不同光下藻类形态变化发现某些丝状蓝藻采用趋向球形形态变小的策略应对红光环境,而采用趋向杆状形态变大的策略适应绿光环境,同时由在此次实验中发现藻类形态参数CPA与Y(NPQ)的正相关关系推测丝状蓝藻在不同光环境下形态上的变化导致的Y(NPQ)变化可能影响丝状蓝藻在水体中的空间分布。
-
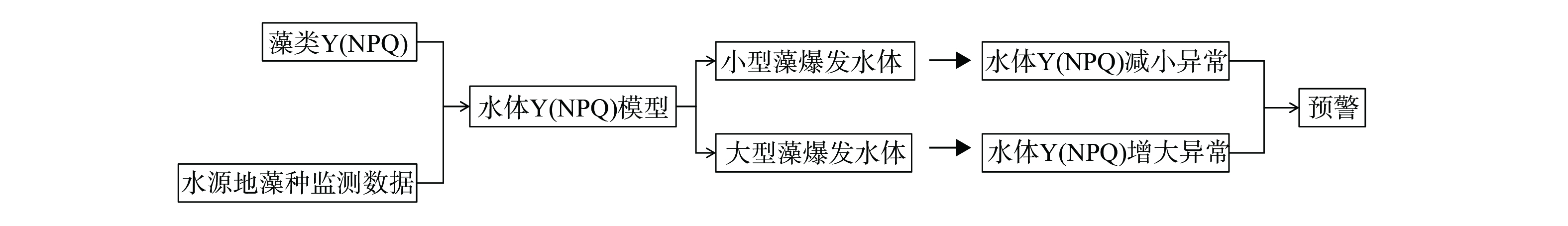
我国水源地众多,不同形态藻类引发的水质问题差异极大,基于有害藻类的形态特征及其与光合特性参数Y(NPQ)的相关性关系,以及于桥水库的数据系统梳理出以水体Y(NPQ)为核心的有害藻类预警防控体系(图5),以期实现针对水源地不同形态有害藻类的预警。模型与水库环境等具体相关,在其他水库需要进一步优化验证。1) 基于已测得水源地典型藻种Y(NPQ)数据以及水库藻种监测数据得到水源地藻种Y(NPQ)时空分布模型,对水体的Y(NPQ)进行常规监测。2) 针对存在硅藻等大型藻类暴发问题的水源地,以于桥水库大型藻类占比最高的冬季藻类Y(NPQ)均值0.074作为预警值,当水体Y(NPQ)值异常增加并维持在0.074以上时预警大型藻暴发。3) 面对微囊藻与丝状藻暴发问题严重的水源地,可以在水体不同深度采样监测水体Y(NPQ),以于桥水库小型藻类占比最高的夏季水体表层藻类Y(NPQ)均值 (0.058) 及水体底层藻类Y(NPQ)均值 (0.051) 作为预警值,当水体Y(NPQ)减小异常并维持在预警值以下时根据采样深度预警微囊藻或丝状藻的暴发。
-
1) 通过系统测定45个藻种形态和光合特征参数,总结形态和光合特征参数的分布规律,建立了藻类形态参数细胞投影面积CPA与光合特性参数Y(NPQ)、α、ETRmax间的正相关线性关系模型。2) 通过监测于桥水库藻类Y(NPQ)变化趋势发现与藻种形态季节性变化相同,不同季节藻类Y(NPQ)均随着水体深度增加而减小,Y(NPQ)可以影响藻类种群的时空分布。3) 模型可应用于防控丝状藻与微囊藻暴发的水体,并为水源地有害藻类控制提供理论支持。
Research on the spatiotemporal distribution mechanism and early warning prevention and control of algal populations in water sources based on algal cell morphology and photosynthetic characteristics
- Received Date: 24/05/2023
- Available Online: 26/10/2023
-
Key words:
- morphology /
- photosynthetic parameters /
- algal /
- spatiotemporal distribution
Abstract: The aim of this study is to clarify the role of cell morphology, the most basic property of algal cells, in the spatiotemporal distribution and competitive succession of algae species. Therefore, 45 common algae species with different cell morphological characteristics from alga, such as cyanobacteria and green algae were selected in this study. The correlation between algal cell morphological characteristics and photosynthetic characteristics was analyzed in the laboratory, and the driving factors of the spatial and temporal distribution of algal morphology were explored based on the algae population monitoring data of Yuqiao Reservoir. It was found that the projected cell area (CPA), which reflected the light-catching potential of algal cells during photosynthesis, was a key morphological parameter affecting photosynthetic characteristics of algal species. By constructing the relationship model between CPA and the regulatory energy dissipation quantum yield (Y(NPQ)), initial slope of photoresponse curve (α), maximum electron transport rate (ETRmax) and other photosynthetic parameters in photosystem II, it is found that there is a strong positive correlation between CPA and Y(NPQ) (R2 = 0.31±0.01, p < 0.001), Y(NPQ) was the key parameter affecting the spatial and temporal distribution of algae population. The comprehensive Y(NPQ) of the algae population in Yuqiao Reservoir showed significant seasonality and gradually decreased from spring to summer. The comprehensive Y(NPQ) of the algae population in summer was the lowest (0.057±0.075), and it showed an increasing trend in autumn and winter. The comprehensive Y(NPQ) of the algae population in winter was the highest (0.072±0.062). In addition, Y(NPQ) of algae population decreased with the increase of water depth, and Y(NPQ) was an important factor affecting the spatial distribution of algae morphology. Based on the data of Yuqiao Reservoir, this study developed an early warning and control system for harmful algae with water body Y(NPQ) as the core, in order to realize the early warning of different forms of harmful algae in water sources.






 DownLoad:
DownLoad:
