-
近年来,由于药品和个人护理产品已成为一种新兴污染物,对人类健康和生态系统存在巨大潜在危害,越来越多的人开始关注此问题[1]。在其中,抗生素因其优良的抗细菌感染特性而被广泛应用于临床医疗。据世界卫生组织调查,我国抗生素人均消耗量是英国和北欧等大部分地区的近6倍[2]。由于人体无法完全代谢和吸收,消耗的大部分抗生素被大量排放至水体环境中。抗生素的滥用不仅会对环境造成污染,而且会促使细菌群体产生抗性导致“超级细菌”的产生[3]。氯霉素作为第一种人工全合成的抗生素类药品,由于对革兰氏阳性菌的作用性较强,曾经被广泛用于各种敏感菌感染的治疗。然而,氯霉素被人体摄入后,有30%~90%的氯霉素被随尿液或粪便排出体外,进入城市污水处理厂[4]。随着使用量的不断增加,氯霉素在土壤、地表水和地下水中均被检测到,甚至在动物体内也含有一定浓度的氯霉素[5]。有研究表明,氯霉素会对人体的消化系统和神经系统产生严重的毒副作用,过量的情况下可引发障碍性贫血和骨髓抑制等致命性疾病[6]。传统污水处理工艺很难将氯霉素完全降解,其中的氯化消毒过程中氯霉素的残留会导致具有“三致”效应消毒副产物的生成,其毒性远远超过母体物质,严重影响饮用水安全[7]。故而寻找一种稳定高效的氯霉素去除方法迫在眉睫。
高级氧化工艺在难降解有机污染物降解方面显示出明显的优势[8-9]。其中,电化学氧化工艺是一种极具吸引力的高级氧化工艺技术,其拥有环境友好、操作简单、可靠性高、适应性广等诸多优点,在新兴污染物如药品和个人护理产品的去除方面展示了优异的性能[10]。电化学氧化技术主要依靠阳极直接或间接氧化污染物的原理进行反应,因此,阳极的选择成为该技术的关键。不同的阳极材料将导致氧化反应的产物、机理以及电流效率的差异[11]。亚氧化钛电极由于具有较高的析氧电位(oxygen evolution potential, OEP)、良好的导电能力、电化学活性以及稳定性等特点,成为了近年来研究较多的电化学阳极材料[12-13]。有研究表明,以Ti4O7为阳极的电化学氧化工艺能够有效地降解氯霉素[14]。相比传统不具备活性的阳极材料(BDD、掺杂SnO2和PbO2等),Ti4O7阳极材料兼具原料储量丰富、低成本和易于制备的优点。尽管如此,原始的Ti4O7具有较低的界面电荷转移率,这将导致其无法提供足够的羟基自由基(·OH)[15-17]。因此,开发一种基于界面改性策略的新型Ti4O7阳极以提高其电催化活性是必要的。
目前,Ti4O7电极主要分为涂层电极和一体式电极。Ti4O7涂层电极主要通过在基体上沉积或是涂覆Ti4O7涂层而制得;Ti4O7一体式电极主要是通过Ti4O7粉末压制后再烧结成型而制备[18]。包括大部分对Ti4O7电极改性的研究也是基于此2种制备方式进行探索。Ti4O7电极较为成功的改性包括WANG等[19]采用等离子喷涂法,将熔化的Ti4O7粉末喷涂在钛板表面制备了Ti/Ti4O7电极,其具有较高的析氧电位,显示出良好的氧化降解能力;NAYAK等[20]在0.5 g Ti4O7粉末中加入2%~3%的石蜡油黏结剂,制备了整体电极,该电极对邻苯二甲酸和对苯二甲酸具有较高的去除率。这些制备Ti4O7电极及改性的方法虽能很好的提高Ti4O7电极的电催化能力,但存在制备方法复杂,稳定性较差的缺点。因此,需要寻找一种可以解决这些问题的改性策略,使得制备工艺简便,得到的电极稳定性优异的同时还可以提高Ti4O7电极电催化能力。
氯霉素通常在污水厂中的检测质量浓度级别为ng·L−1或μg·L−1[21],但在实验室中,为了凸显电催化性能的降解能力,通常选用mg·L−1的质量浓度作为模拟氯霉素废水,如杨志伟等[22]用超声强化Ti4O7电极,在最佳反应条件下,对初始质量浓度为20 mg·L−1的氯霉素去除率为82.11%。本研究选用20 mg·L−1的氯霉素模拟废水作为目标污染物,采用缺陷工程方法制备Zr、钇(yttrium, Y)和锰(manganese, Mn)不同过渡金属元素掺杂的Ti4O7电极,探讨这些改性Ti4O7电极对于氯霉素的去除效能,重点比较Zr/Ti4O7阳极和纯Ti4O7阳极对氯霉素的电化学降解性能,通过微观结构、元素分析和电化学测试等对Zr元素掺杂亚氧化钛(Zr/Ti4O7)阳极的电极性能进行表征并通过循环实验测试了Zr/Ti4O7阳极的稳定性。重点优化研究初始电流密度、初始pH,常见阴离子等因素影响下Zr/Ti4O7阳极对氯霉素的降解并分析氯霉素的降解机理,此外,还将进一步探究Zr/Ti4O7作为阳极的电化学氧化工艺对不同抗生素的降解能力,以期为改性Zr/Ti4O7电极对氯霉素的降解提供参考。
-
高纯度(>95%)Ti4O7粉购自山东联盟化工集团有限公司;氯霉素,纯度大于97%,购自阿拉丁生化科技股份有限公司;硝酸锆,纯度大于99%,购自上海麦克林化学试剂有限公司;其余实验药品均为分析纯,购自上海麦克林化学试剂有限公司,使用前未做进一步纯化处理;实验用水是经纯水机(Millipore)制备的超纯水,电阻率为18 MΩ·cm。
-
取2 g Ti4O7粉末,将粉末放入直径为2 cm的圆柱形石墨模具内,置于真空等离子烧结炉中烧制,得到直径为2 cm的Ti4O7电极片[23]。将Ti4O7电极进行真空等离子体处理,真空等离子处理放电功率为100 W,处理气体为氩(argon, Ar)气,处理时间为30 min,得到缺陷Ti4O7电极。将缺陷Ti4O7电极片浸入50 mL去离子水中室温超声1 h。而后在去离子水中加入0.4 g Zr(NO3)4粉末,搅拌12 h使缺陷Ti4O7电极充分吸附Zr离子,随后取出电极用去离子水清洗,冷冻干燥24 h。将干燥后的电极在5%H2/Ar混合气氛中,以5 ℃·min−1升温速率在200 ℃热处理2 h,得到电极片命名为Zr/Ti4O7。该相同改性方法下,将Zr(NO3)4粉末分别换成Y(NO3)3和Mn(NO3)2,其余条件如处理时间、处理温度等都相同,分别得到Y/Ti4O7和Mn/Ti4O7阳极。
-
电极形貌结构和表面元素组成采用捷克TESCAN MIRA LMS扫描电子显微镜(scanning electron microscope, SEM)进行分析,电极表面晶型和强度用日本Ultima IV公司的EMPYREAN衍射仪进行X射线衍射(X-ray diffractometer, XRD)表征。对制备的Zr/Ti4O7阳极和纯Ti4O7阳极进行线性伏安扫描(linear sweep voltammetry, LSV)和循环伏安扫描(cyclic voltammetry, CV),测试采用电化学工作站(Auto Lab M204型,上海辰华三电极体系:参比电极为 Ag/AgCl(饱和KCl)),对电极为铂片,工作电极为制备的电极。
-
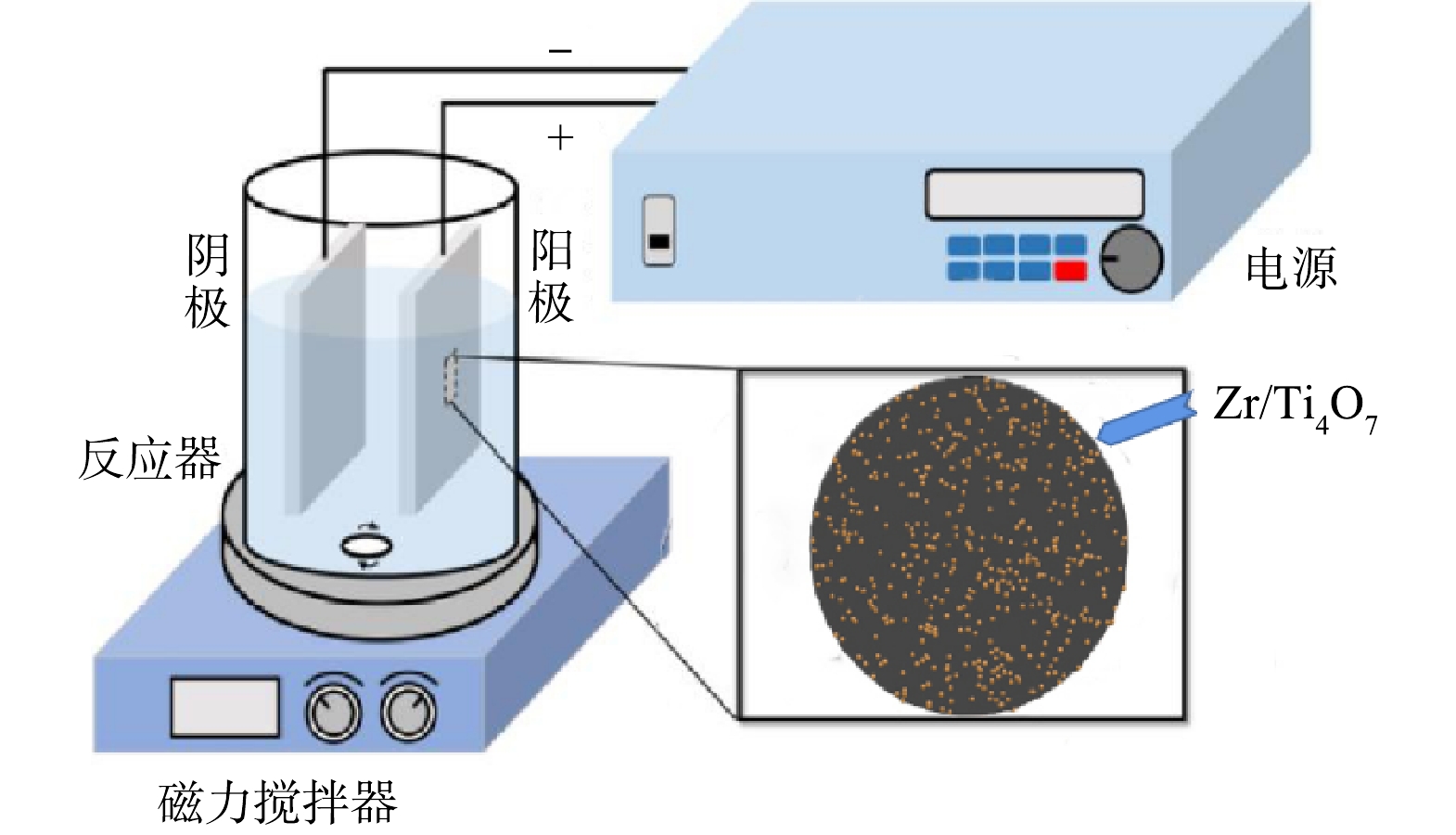
电化学降解过程,在室温下进行,装置见图1。Zr/Ti4O7或Ti4O7作为阳极,钛板作为阴极,电源采用直流稳压电源(大华无线,中国)。电化学催化氧化反应过程中,电极垂直浸入氯霉素水溶液中,阳极和阴极板之间的电极间距保持在1 cm。实验在100 mL的玻璃烧杯中进行,室温(298±1) K,磁力搅拌器转速为200 r·min−1。电解液体积为100 mL, 电解质Na2SO4浓度为100 mmol·L−1, 氯霉素质量浓度为20 mg·L−1。除非另有规定,否则不调整氯霉素反应溶液的pH。将电极插入反应溶液中,并连接到直流电源以调节功率。浸泡在溶液中的平板总面积为3.14 cm2, 接通直流电源立即启动反应。在整个反应过程中,在预定的时间间隔内,用注射器抽出样品,并立即在0.45 μm聚四氟乙烯膜上过滤。用高效液相色谱进行分析,所有样品至少测量2次。
-
实验采用高效液相色谱法(Agilent USA)测定氯霉素浓度。具体检测条件为:采用G1 365MWD紫外检测器;色谱柱为Eclipse XDB C18(5 μm,4.6 mm×250 mm);设置高效液相色谱仪检测波长为275 nm;柱温为30 ℃;流动相采用的是水∶甲醇=45∶55;流速为0.8 m L·min−1;进样量为10 μL。
不同实验参数下的每阶电能(electric energy per order, EEO)由式(1)计算得出。
式中:EEO为每阶电能,kWh·m−3;P为电催化氧化体系中电流产生的实际功率,kW;t为反应时间,min;C0和Ct为0和t时氯霉素的质量浓度,g·L−1;V为反应液体积,L。
-
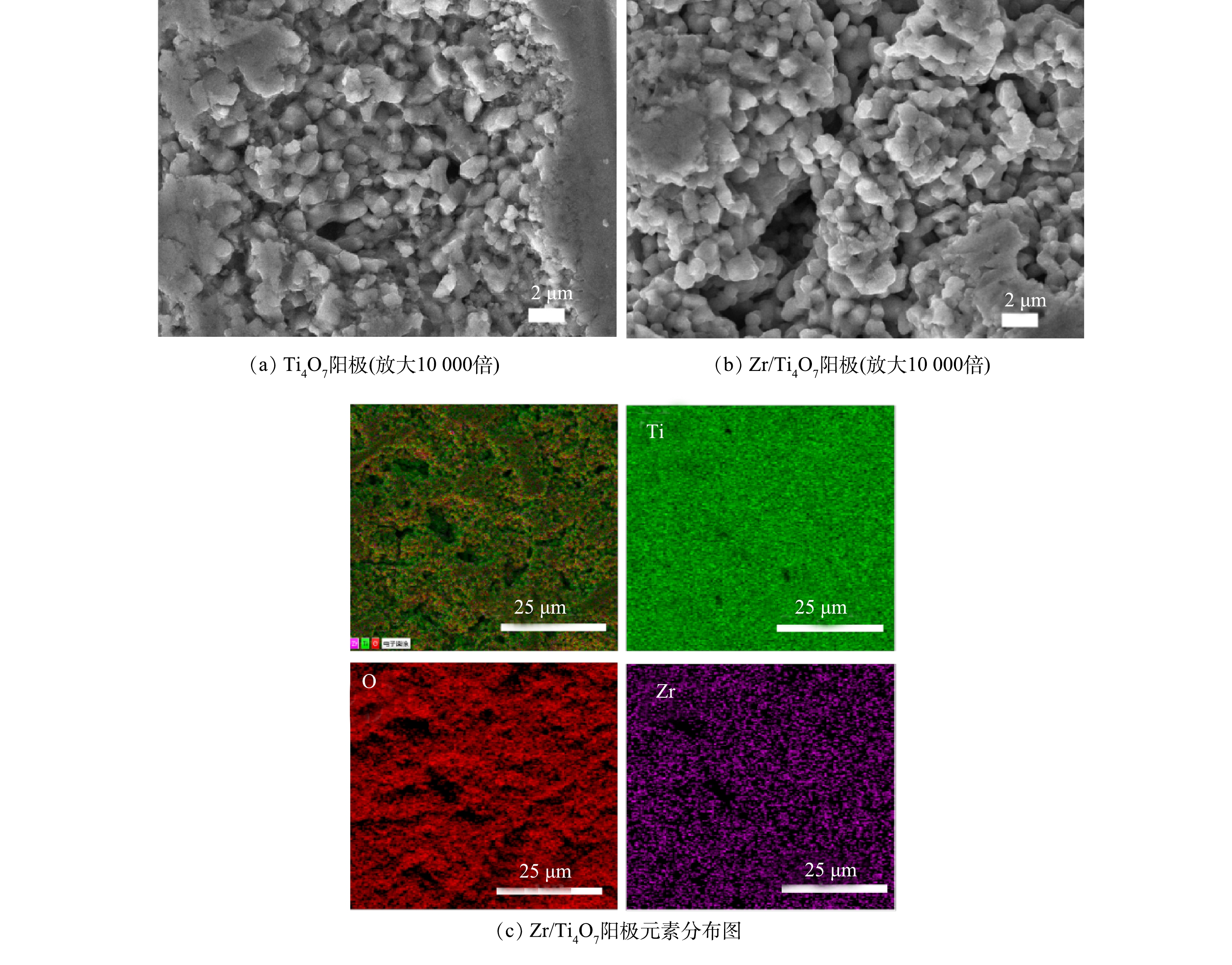
1)图2为Ti4O7阳极和Zr/Ti4O7阳极在10 000倍下的SEM图。由图2中可以很明显的看出Ti4O7粉末颗粒在真空等离子烧结后颗粒熔融并且聚集在一起形成整体,纯Ti4O7阳极表面呈现出由熔融颗粒组成的致密结构(图2(a))。由图2(b)可以看出,纯Ti4O7电极在经过Ar气等离子体处理及Zr元素掺杂后,Ti4O7电极表面出现了明显的蚀刻现象。根据目前的研究报道,等离子体处理可以在Ti4O7电极表面产生氧空位,为后续掺杂Zr元素奠定基础[24]。由元素分布图可以看出(图2(c)),Zr/Ti4O7阳极表面主要组成元素是Zr、Ti和O元素,并且这些元素在Zr/Ti4O7阳极表面分布均匀,其原子质量占比分别为0.2%、64.4%和32.5%。
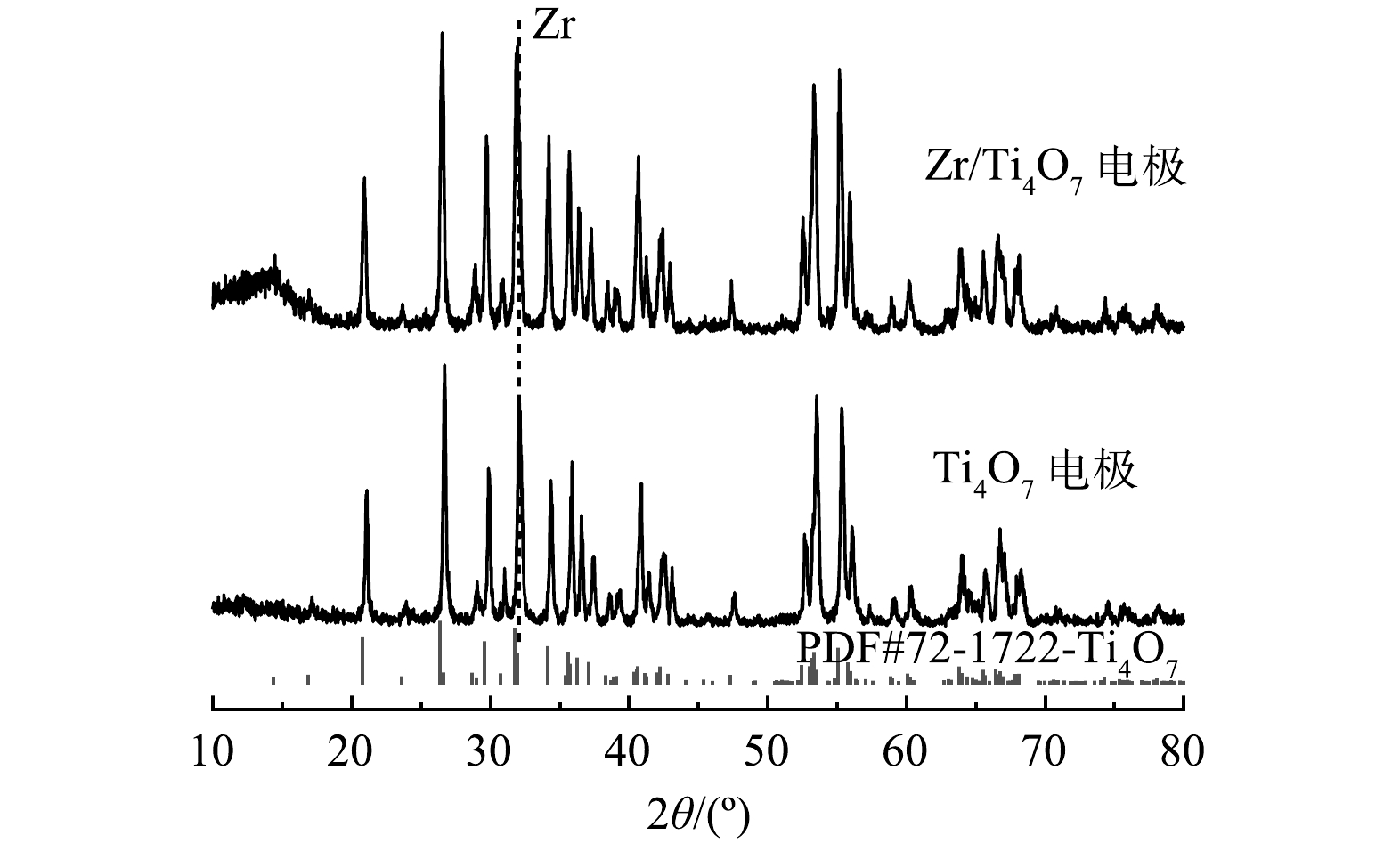
2)由XRD图谱(图3)可以看出,所制备的纯Ti4O7在2θ分别为20.78°、26.38°、29.59°、31.77°、34.10°、53.36°和55.08°等衍射角处均出现明显的特征衍射峰,符合XRD标准卡片谱图(JCPDS 72-1722)中Ti4O7的特征衍射峰;经过Zr掺杂改性后的Ti4O7电极表面的衍射峰在2θ≈31.7°处由于出峰位置出现了重叠,因而未出现新的峰,但峰强度明显有所增大,这证实了在Ti4O7表面成功掺杂了少量Zr元素。此外,通过比较改性前后的Ti4O7的XRD图谱,发现没有新峰出现,且改性前后峰没有明显的移位。这说明除了少量Zr元素掺杂外,其结构和组成基本没有变化。
-
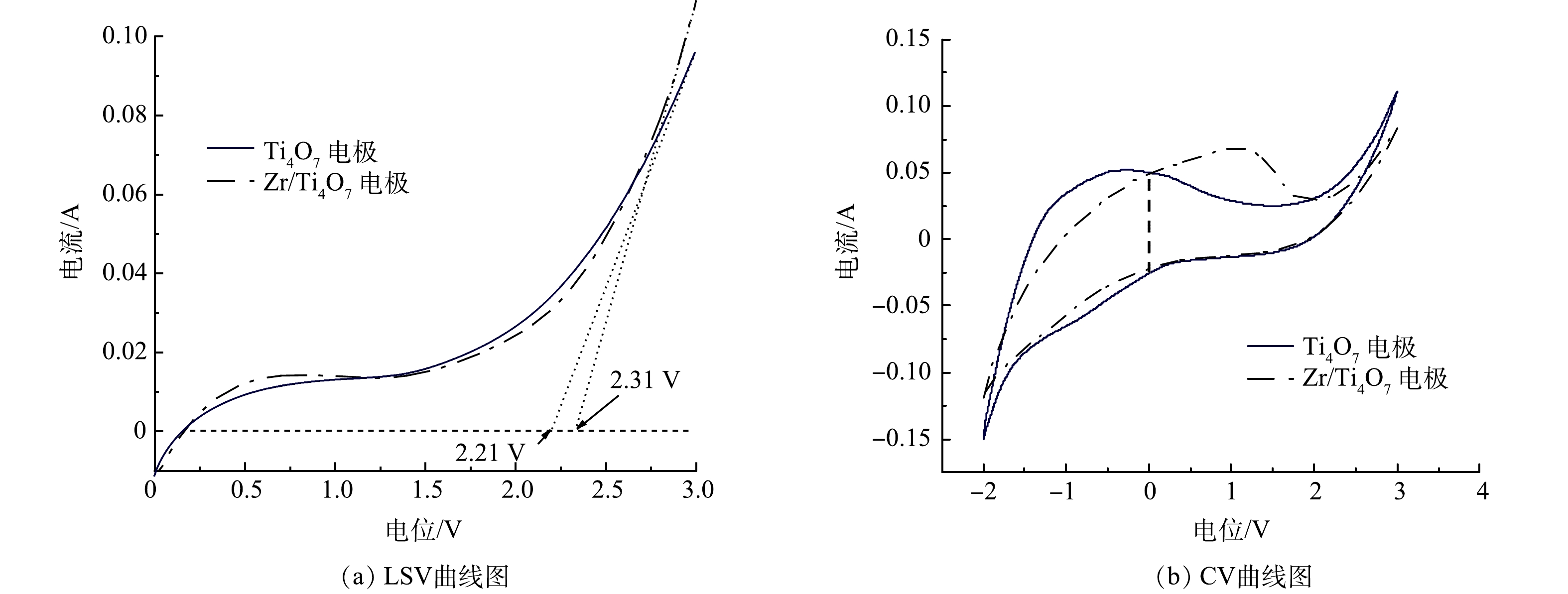
在0.1 mol·L−1 Na2SO4溶液中原始Ti4O7和Zr/Ti4O7电极的LSV光谱如图4(a)所示。结果表明,原始Ti4O7电极和Zr/Ti4O7电极的OEP分别为2.21 V和2.31 V,均接近BDD电极。LSV曲线能反映极板OEP值的变化规律,OEP值越高,电化学析氧副反应越难发生,因而电化学氧化有机物的电流效率越高[25]。Zr/Ti4O7电极的OEP高于纯Ti4O7电极,表明Zr/Ti4O7电极具有更高的水分解电子转移活性和更高的·OH生成速率[26]。图4(b)为纯Ti4O7电极和Zr/Ti4O7电极的CV扫描结果。如图4(b)所示,Zr/Ti4O7电极零电势的虚线与CV测试曲线所构成的闭合曲线代表的电流面积明显大于纯Ti4O7电极CV测试的电流面积,表明改性电极的电子转移活性较原电极增强。由CV测试结果来看,Zr/Ti4O7电极比原Ti4O7电极具有更大的电活性面积,这说明等离子体处理和Zr元素掺杂产生的氧空位在Ti4O7表面可提供额外的反应位点。
-
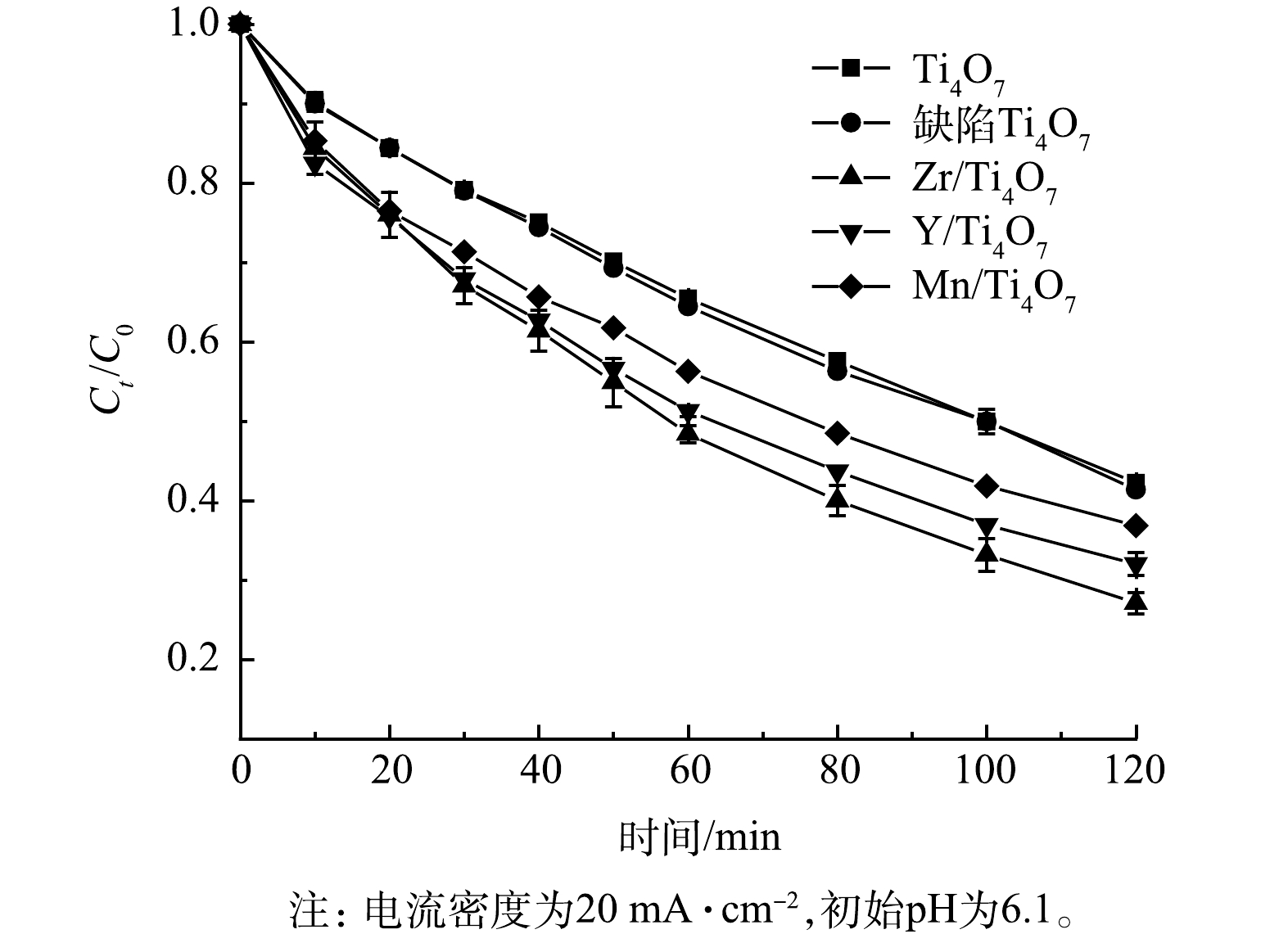
为了评价纯Ti4O7阳极、仅等离子体处理的缺陷Ti4O7阳极及不同过渡金属元素掺杂下的改性Ti4O7阳极在电化学体系下的催化活性,实验在制备Zr/Ti4O7阳极的同时,随机选取了2种过渡金属元素Y和Mn元素,制备了Y/Ti4O7和Mn/Ti4O7阳极。在初始电流密度为20 mA·cm−2、不改变溶液pH(pH=6.1)的条件下,降解2 h,2种电极对于氯霉素的去除率变化情况如图5所示。由图5可以看出,缺陷Ti4O7阳极对于氯霉素的最终去除率为58.6%,与纯Ti4O7阳极的去除效果几乎相同,因而,仅仅等离子体处理并没有实质性提高Ti4O7阳极的电催化性能。而Zr/Ti4O7、Y/Ti4O7、Mn/Ti4O7改性阳极在经过2 h的电氧化反应后,氯霉素的降解率分别为73.8%、69%和63%,优于纯Ti4O7阳极的降解率(57%)。此外,Zr掺杂改性后的阳极对氯霉素的降解率也是优于Y和Mn元素掺杂的Ti4O7阳极。可以发现,Ti4O7阳极在经过等离子体处理及过渡金属元素掺杂后的电催化活性均得到提升,同时,相同条件下的Zr/Ti4O7阳极比纯Ti4O7阳极的降解率提高了接近17%。这说明Zr元素的掺杂使得Ti4O7阳极对污染物的降解能力得到明显提高。该结果也与前面在电化学测量中所提到的Zr/Ti4O7电极相较于纯Ti4O7电极具有更高的析氧电位和更大的电活性面积这一结果相吻合。
有研究[24]表明,等离子体处理和过渡金属元素掺杂可以有效增加Ti4O7电极表面氧空位,从而大大增加·OH等自由基的生成概率,进而可促进污染物的降解。结果表明,Zr过渡金属元素相较于Y和Mn对于Ti4O7阳极具有更好的改性效果。这可能是因为Zr元素可以更好的调整O元素的电子结构从而更容易与O形成化学键,在形成化学键的同时会诱导Ti4O7表面产生氧空位,进而有效提高Ti4O7阳极的电催化活性[27]。
-
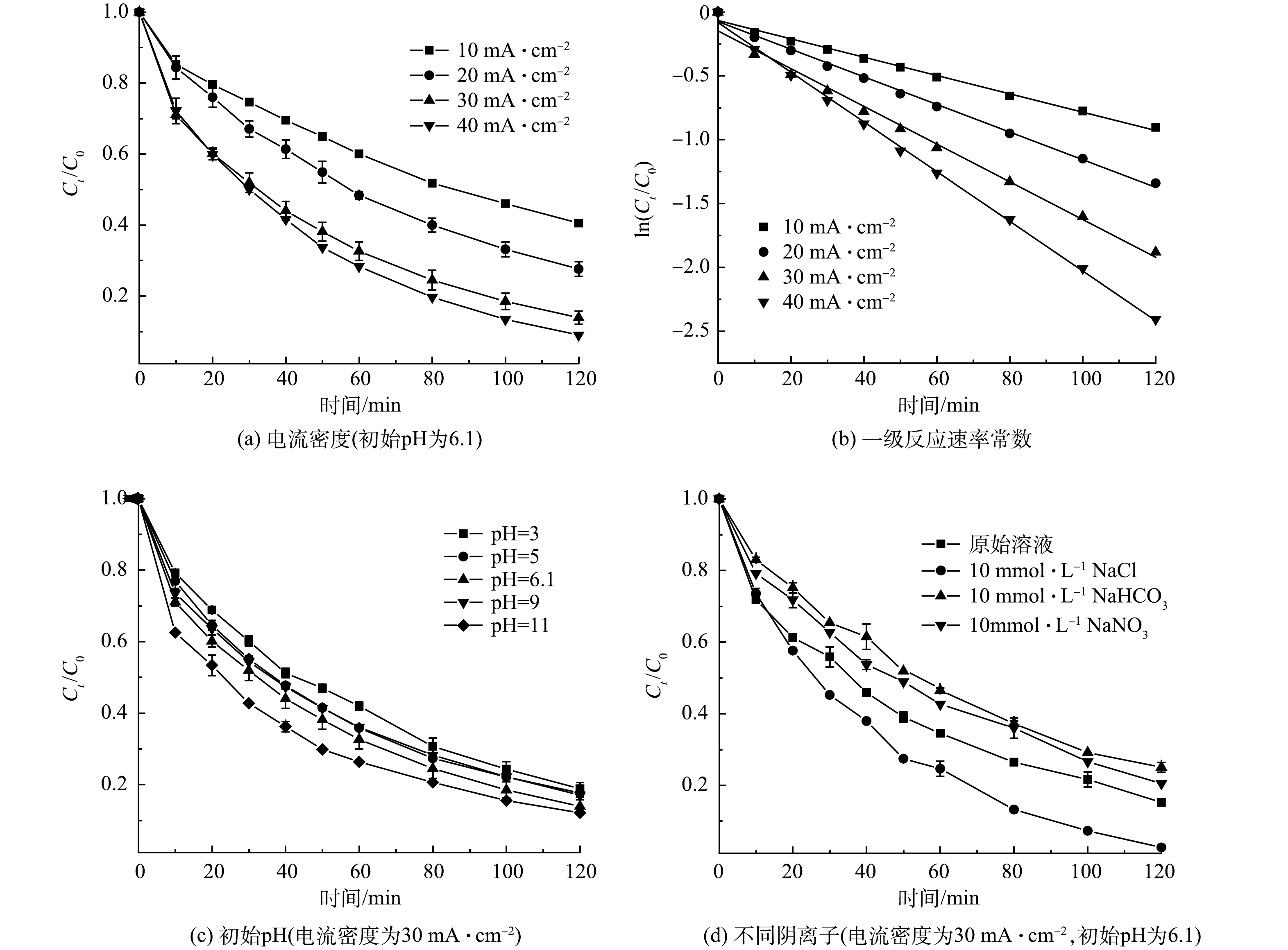
在Zr/Ti4O7阳极电化学降解氯霉素实验中,通过测定不同电流密度、溶液初始pH、溶液中共存阴离子等参数来验证反应过程中氯霉素质量浓度的变化,从而分析氯霉素的去除率变化规律。
本研究考察了当溶液初始pH=6.1,电流密度对氯霉素的降解影响。由图6(a)可以看到,氯霉素的降解率随电流密度的增大而增大。当电流密度由10 mA·cm−2增至40 mA·cm−2时,氯霉素降解率由59.5%增至91%,反应速率常数k由0.007 19 min−1增至0.019 48 min−1(表1)。可见,随着电流密度的增大,氯霉素电化学降解速率加快,并且反应符合一级反应动力学规律(图6(b))。有研究表明,在电化学氧化过程中,·OH的生成速率主要取决于施加的电流密度,电流密度越大,生成的·OH速率越快,从而加速了氯霉素的降解[28]。虽然氯霉素的降解效率随着电流密度的增加而提高,但随之而带来的能耗也有所上升,当电流密度由30 mA·cm−2增至40 mA·cm−2时,降解率增加了6.2%,能耗却增大了3.34 kWh·m−3,相比较下电流密度由20 mA·cm−2增至30 mA·cm−2时的降解率增大11%,能耗仅仅只增大了2.39 kWh·m−3。因此,综合考虑降解效率和能耗问题,后续实验选30 mA·cm−2最佳电流密度。
溶液的初始pH是电化学降解污染物的重要影响因素,也对自由基的产生起重要作用。在电流密度为30 mA·cm−2的条件下,用1mol·L−1的硫酸和氢氧化钠溶液将反应溶液初始pH调整为3、5、9和11,反应2 h后氯霉素的降解情况如图6(c)所示。在溶液初始pH为3~11时,氯霉素的降解率为81.1%~87.9%。可以明显的发现,以改性Ti4O7为阳极、电化学体系在较宽的pH范围内对ATL具有良好的降解效果。同时,由图6(c)中可以看到,酸性条件和弱碱性条件下抑制了氯霉素降解,但在强碱性溶液中,氯霉素的降解率相较于未调节pH之前又有所提高。这可能是溶液在较高的pH下,氯霉素分子容易失去2个可电离的氢原子,以促进亲电·OH的氧化。此外,氯霉素以阴离子的形式存在,更容易吸附在电极表面,有利于电迁移和传质[29]。考虑到调整溶液pH的额外成本以及污染物的最终去除率差异较小,后续研究选择不调节溶液pH。
电化学氧化降解实际废水时,大多数废水中会存在Cl−、HCO3−和NO3−等常见的阴离子,这些阴离子的存在或多或少会对污染物的降解起到影响作用。实验在电流密度为30 mA·cm−2,初始pH为6.1的条件下探究了不同无机阴离子对氯霉素降解的影响。不同无机阴离子的浓度均为10 mmol·L−1,结果如图6(d)所示。反应2 h后,加入Cl−的溶液中氯霉素的去除率达到了97.4%,Cl−的存在有效的增强Zr/Ti4O7阳极对氯霉素的降解能力。这可能是因为,在电化学体系中Cl−容易被氧化成活性氯自由基,从而与·OH一起加速了有机物的阳极氧化分解[30]。相反,HCO3−和NO3−的存在抑制了氯霉素的降解,其中HCO3−的抑制较为明显,这可能是因为HCO3−易与·OH反应生成活性较低的碳酸根自由基,与污染物竞争从而降低了氯霉素的降解率[31]。因此,在以Zr/Ti4O7为阳极的电化学氧化对污染物降解时,可以适当加入氯化钠作为一种合适且有效的辅助电解质。
-
经过检测发现,未通电的情况下,在Zr/Ti4O7阳极加入反应溶液前后,污染物浓度几乎没有变化,说明阳极对该污染物没有显著的吸附作用。因此,电催化降解体系中氯霉素的降解主要通过自由基的氧化和电极的直接电子转移实现。众所周知,叔丁醇是·OH的清除剂,对苯醌是超氧自由基(O2·−)的清除剂,而甲醇是大多数自由基的清除剂[32]。本实验采用叔丁醇、对苯醌和甲醇淬灭电催化体系降解氯霉素,探索反应体系中主要的自由基。在不添加清除剂的情况下,反应2 h后,氯霉素的降解率为86.1%。而加入0.05 mol·L−1叔丁醇后,反应2 h降解率降低至46%。同样,当添加5.4 g对苯醌时,氯霉素的降解率降低至68%。最后,当添加0.05 mol·L−1甲醇时,氯霉素的降解率被强烈抑制,仅为12%左右。从淬灭实验结果可以看出,·OH和O2·−对氯霉素的降解起主要作用,反应2 h后,·OH和O2·−对氯霉素降解的贡献率分别约为40%和18%。直接电子转移贡献率约占12%,而剩下约16%的降解率可能是存在·OH和O2·−的协同作用或其他未知自由基贡献的(图7)。
-
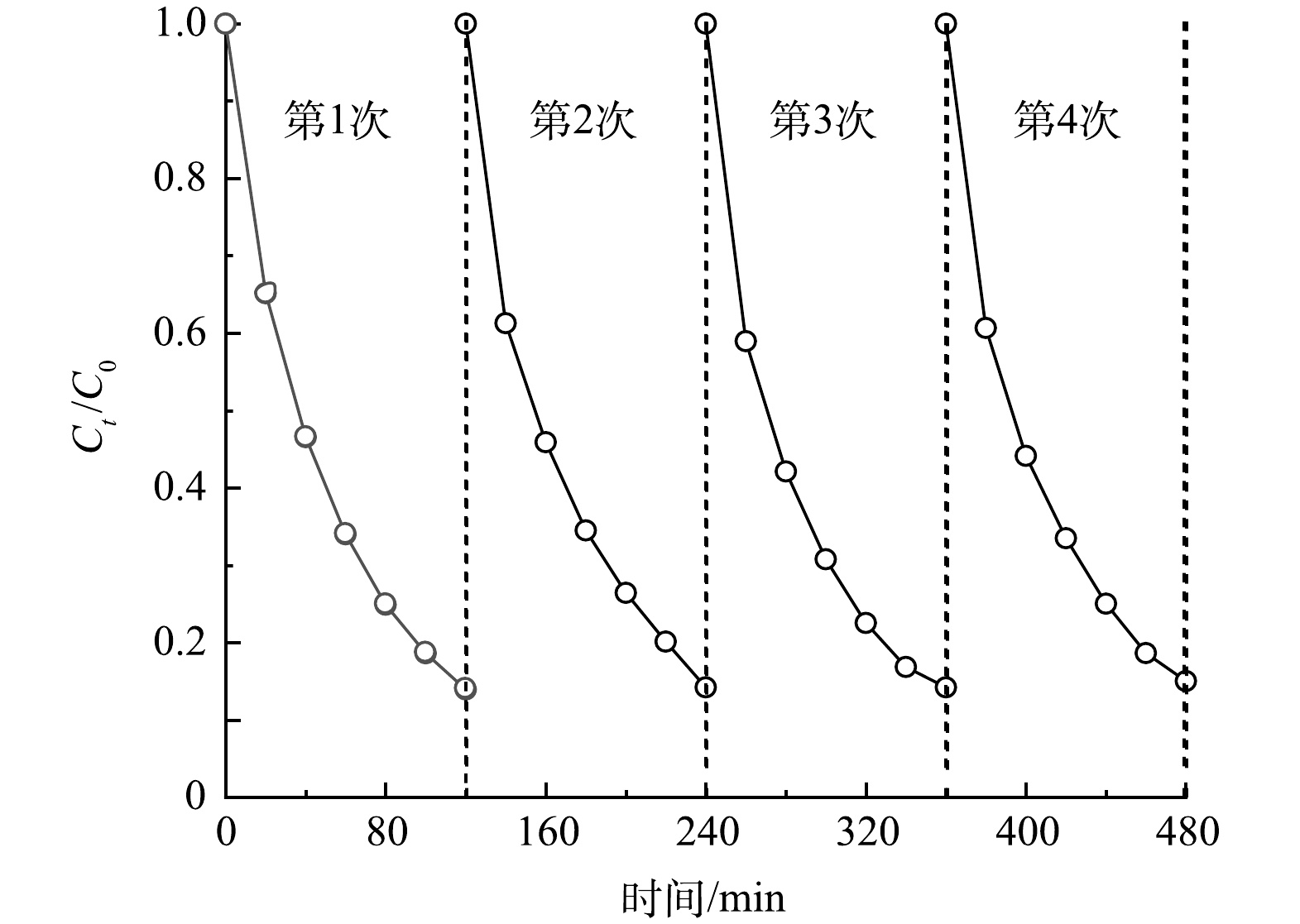
在电催化降解体系中进一步考察了Zr/Ti4O7阳极的稳定性。由图8可知,在电流密度为30 mA·cm−2、初始溶液pH为6.1的条件下,反应2 h后,氯霉素去除率达到86.1%左右。在相同的实验条件下对反应洗净后的Zr/Ti4O7阳极进行了4次循环实验。由图8可以看出,在4次循环实验中,Zr/Ti4O7阳极对氯霉素的降解率分别约为86%、85.8%、85.8%和85%。由此可见,改性电极对氯霉素的去除率几乎没有变化,在简便制备工艺下得到的改性电极稳定性优异,具有大规模工业应用的潜力。
-
上述结果证明利用Zr/Ti4O7阳极的电催化氧化可以实现对模拟氯霉素废水的快速降解。为进一步评估Zr/Ti4O7阳极对药物类废水降解的通用性,另外选取了一种抗生素类药物氟苯尼考和一种解热镇痛的抗炎药双氯芬酸钠,在最优反应参数条件下考察了他们的降解效能(图9)。反应2 h后,氟苯尼考和双氯芬酸钠的降解率分别达到93.4%和85.5%,这说明以Zr/Ti4O7作为阳极的电氧化同样可以高效降解氟苯尼考和双氯芬酸钠。可见,Zr/Ti4O7阳极用于电化学降解药品和个人护理产品这一类新兴污染物具有实用性。
综上所述,Zr/Ti4O7阳极对于药物类污染物具有优异的电氧化活性,尤其是在最优条件下对于氯霉素可以达到97.4%的降解率。相比较之下,使用载铁活性炭颗粒电极作为阳极降解氯霉素,在反应2 h候氯霉素的去除率仅有74.17%(表2)。另一种使用Ti/SnO2-Sb-Ni电极电氧化氯霉素虽然降解率可超过99%,但反应时间却需要300 min。同样还包括新型Al掺杂PbO2电极,其对于氯霉素的去除率也低于Zr/Ti4O7阳极。表2还反映了不同电极电氧化氟苯尼考和双氯芬酸钠的降解率。综合来看,Zr/Ti4O7阳极的电化学氧化性能较好。
-
1) Zr、Y、Mn元素掺杂的改性Ti4O7阳极对氯霉素的降解率分别为73.8%、69%和63%。与纯Ti4O7阳极(57%)相比可以发现,该改性工艺制备下的过渡金属元素掺杂的Ti4O7电极具有比原电极更优异的电催化性能。2)与纯Ti4O7阳极相比,采用过渡金属元素Zr掺杂制备的Zr/Ti4O7阳极对水中氯霉素的去除效果最佳。在电流密度为30 mA·cm−2,初始pH为6.1,电解质为Na2SO4(100 mmol·L−1)和少量NaCl (10 mmol·L−1)时,Zr/Ti4O7阳极对氯霉素降解率最高,为97.4%。通过对其进行SEM、XRD及电化学性能的表征可知,该电极表面具有丰富的氧空位,具备较高的析氧过电位和优异的电化学活性等优点。3) Zr/Ti4O7阳极对氯霉素具有良好的降解效果得益于自身的电子转移以及溶液中·OH和O2·−对于污染物的氧化,电氧化阳极产生的·OH是氯霉素去除的主要氧化自由基。4)改性电极具有优异的稳定性,同时该阳极对氟苯尼考和双氯芬酸钠等药物模拟废水同样具有优异的降解性能,在最佳条件下其降解率分别能达到93.4%和85.5%。
改性Ti4O7阳极对氯霉素的高效电氧化降解
Highly efficient electrooxidation degradation of chloramphenicol by modified Ti4O7 anode
-
摘要: 针对传统电化学氧化中阳极是污染物催化氧化的核心,寻找一种具有高析氧电位,高反应活性面积,优良催化活性的阳极是当下亟待解决的问题。为此,选取了亚氧化钛(Ti4O7)电极作为基础电极且对其进行过渡金属元素掺杂改性。通过各种表征及降解性能研究发现,所制备改性电极相对于纯Ti4O7电极催化性能有所提高。其中,锆(zirconium, Zr)元素掺杂后使得改性电极具有更高的析氧电位和更好的电催化活性。在电流密度为30 mA·cm−2,初始pH为6.1,电解质为100 mmol·L−1 Na2SO4和10 mmol·L−1 NaCl的最优条件下,氯霉素的去除率为97.4%。并且该电极具有很好的抗干扰性和稳定性,在不同pH干扰下对氯霉素依然具有较高的降解率(均高于81.1%)。此外,Zr元素掺杂的Ti4O7改性电极对氟苯尼考和双氯芬酸钠等药物也具有很好的降解效果,降解率分别为93.4%和85.5%,本研究结果可为电化学去除污染物阳极改性研究提供参考。
-
关键词:
- 电化学氧化 /
- Zr/Ti4O7阳极 /
- 掺杂 /
- 氧空位 /
- 氯霉素
Abstract: Considering that anode is the core of catalytic oxidation of pollutants in traditional electrochemical oxidation, it is urgent to find an anode with high oxygen evolution potential, high reactivity area and excellent catalytic activity. In this study, titanium oxide (Ti4O7) electrode was selected as the base electrode and modified by doping the transition metal element. Various characterization and degradation experiments showed that the catalytic ability of the modified electrode increased compared with that of pure Ti4O7 electrode. Of which, Zirconium (Zr) doping increased the oxygen evolution potential and electrocatalytic activity. Under the optimal conditions: current density of 30 mA·cm−2, initial pH 6.1, electrolyte of 100 mmol·L−1 Na2SO4 and 10 mmol·L−1 NaCl, the removal rate of chloromycin was 97.4%. Moreover, the electrode had a good anti-interference and stability, and still had excellent degradation rates of chloromycin under different pH interference (all higher than 81.1%). At the same time, zirconium-doped Ti4O7 modified electrode also had a good degradation ability to flufenicol or diclofenac sodium, their degradation rate reached 93.4% and 85.5%, respectively. This study provides a reference for the electrochemical removal of pollutants and anode modification research.-
Key words:
- electrochemical oxidation /
- Zr/Ti4O7 anode /
- doping /
- oxygen vacancy /
- chloramphenicol
-

-
表 1 在不同电流密度下Zr/Ti4O7阳极降解氯霉素的反应动力学常数及能耗
Table 1. Kinetic constants and energy consumption of chloramphenicol degradation by Zr/Ti4O7 anode at different current densities
电流密度/
(mA·cm−2)降解率/% k/min−1 R2 EEO/
(kWh·m−3)10 59.5 0.0071 9±0.000 2 0.991 7.25 20 73.8 0.0108 3±0.000 2 0.995 10.6 30 86.1 0.0147 9±0.000 5 0.990 12.99 40 91.0 0.0194 8±0.000 3 0.998 16.33 表 2 不同电氧化处理工艺对药物类有机物降解率的比较
Table 2. Comparison of degradation rates of medicated organic compounds by different electrooxidation processes
-
[1] KUMAR A, KHAN M, HE J, et al. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review[J]. Water Research, 2020, 170: 115356. doi: 10.1016/j.watres.2019.115356 [2] QIAO M, YING G-G, SINGER A C, et al. Review of antibiotic resistance in China and its environment[J]. Environment International, 2018, 110: 160-172. doi: 10.1016/j.envint.2017.10.016 [3] BAILóN-PéREZ M I, GARCíA-CAMPAñA A M, CRUCES-BLANCO C, et al. Trace determination of β-lactam antibiotics in environmental aqueous samples using off-line and on-line preconcentration in capillary electrophoresis[J]. Journal of Chromatography A, 2008, 1185(2): 273-280. doi: 10.1016/j.chroma.2007.12.088 [4] HEBERER T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data[J]. Toxicology Letters, 2002, 131(1-2): 5-17. doi: 10.1016/S0378-4274(02)00041-3 [5] LIN Y C, YU T H, LIN C F. Pharmaceutical contamination in residential, industrial, and agricultural waste streams: Risk to aqueous environments in Taiwan[J]. Chemosphere, 2009, 74(1): 131-141. [6] 唐玉霖, 曲鑫璐, 刘倩宏, 等. 紫外联用高级氧化技术降解氯霉素试验研究[J]. 同济大学学报(自然科学版), 2021, 49(09): 1249-1256. [7] CHU T, YANG D, CHU W, et al. Increased formation of halomethanes during chlorination of chloramphenicol in drinking water by UV irradiation, persulfate oxidation, and combined UV/persulfate pre-treatments[J]. Ecotoxicology and Environmental Safety, 2016, 124: 147-154. doi: 10.1016/j.ecoenv.2015.10.016 [8] WANG A, GUO S, ZHENG Z, et al. Highly dispersed Ag and g-C3N4 quantum dots co-decorated 3D hierarchical Fe3O4 hollow microspheres for solar-light-driven pharmaceutical pollutants degradation in natural water matrix[J]. Journal of hazardous materials, 2022, 434: 128905. doi: 10.1016/j.jhazmat.2022.128905 [9] WANG A, CHEN Y, ZHENG Z, et al. In situ N-doped carbon-coated mulberry-like cobalt manganese oxide boosting for visible light driving photocatalytic degradation of pharmaceutical pollutants[J]. Chemical Engineering Journal, 2021, 411: 128497. doi: 10.1016/j.cej.2021.128497 [10] 赵瑞. 电化学法降解抗生素类有机物的研究[D]. 济南: 齐鲁工业大学, 2019. [11] 张思韬, 韩严和, 张晓飞, 等. 用于处理工业废水的电极材料研究进展[J]. 工业水处理, 2019, 3 9(11): 1-6. [12] 智丹, 王建兵, 王维一, 等. Ti/Ti4O7阳极电化学氧化降解水中的美托洛尔[J]. 环境科学学报, 2018, 38(5): 1858-1867. [13] 韩金名. NF/CN-TF/Ti4O7电化学体系对磺胺甲基嘧啶降解机制研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. [14] 王愚. 亚氧化钛电化学阳极氧化降解印染废水研究[D]. 哈尔滨: 哈尔滨工业大学, 2016. [15] XIE J, MA J, ZHANG C, et al. Effect of the Presence of Carbon in Ti4O7 Electrodes on Anodic Oxidation of Contaminants[J]. Environmental Science and Technology, 2020, 54(8): 5227-5236. doi: 10.1021/acs.est.9b07398 [16] BEJAN D, GUINEA E, BUNCE N J. On the nature of the hydroxyl radicals produced at boron-doped diamond and Ebonex anodes[J]. Electrochimica Acta, 2012, 69: 275-281. doi: 10.1016/j.electacta.2012.02.097 [17] GAYEN P, CHEN C, ABIADE J T, et al. Electrochemical oxidation of atrazine and clothianidin on Bi-doped SnO2-TinO2n-1 electrocatalytic reactive electrochemical membranes[J]. Environmental Science and Technology, 2018, 52(21): 12675-12684. doi: 10.1021/acs.est.8b04103 [18] 杨泽坤, 刘永, 杨海涛等. 亚氧化钛电极的制备及其在废水处理中的应用[J]. 工业水处理, 2022, 42(11): 56-64. doi: 10.19965/j.cnki.iwt.2021-1118 [19] WANG H, LI Z, ZHANG F, et al. Comparison of Ti/Ti4O7, Ti/Ti4O7-PbO2-Ce, and Ti/Ti4O7 nanotube array anodes for electro-oxidation of p-nitrophenol and real wastewater[J]. Separation and Purification Technology, 2021, 266: 118600. doi: 10.1016/j.seppur.2021.118600 [20] NAYAK S, CHAPLIN B P. Fabrication and characterization of porous, conductive, monolithic Ti4O7 electrodes[J]. Electrochimica Acta, 2018, 263: 299-310. doi: 10.1016/j.electacta.2018.01.034 [21] 张立宝. Ti/SnO2-Sb-Ni电极制备及对CAP废水的降解机理研究[D]. 沈阳: 沈阳工业大学, 2020. [22] 杨志伟. 超声强化亚氧化钛阳极电化学氧化处理氯霉素废水的研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. [23] LIN H, NIU J, LIANG S, et al. Development of macroporous Magnéli phase Ti4O7 ceramic materials: as an efficient anode for mineralization of poly- and perfluoroalkyl substances[J]. Chemical Engineering Journal, 2018, 354: 1058-1067. doi: 10.1016/j.cej.2018.07.210 [24] ZHU K, SHI F, ZHU X, et al. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction[J]. Nano Energy, 2020, 73: 104761. doi: 10.1016/j.nanoen.2020.104761 [25] LI D, TANG J, ZHOU X, et al. Electrochemical degradation of pyridine by Ti/SnO2–Sb tubular porous electrode[J]. Chemosphere, 2016, 149: 49-56. doi: 10.1016/j.chemosphere.2016.01.078 [26] SäRKKä H, BHATNAGAR A, SILLANPää M. Recent developments of electro-oxidation in water treatment: A review[J]. Journal of Electroanalytical Chemistry, 2015, 754: 46-56. doi: 10.1016/j.jelechem.2015.06.016 [27] JIAO T, LU C, ZHANG D, et al. Bi-functional Fe2ZrO5 modified hematite photoanode for efficient solar water splitting[J]. Applied Catalysis B:Environmental, 2020, 269: 118768. doi: 10.1016/j.apcatb.2020.118768 [28] 于丽花, 薛娟琴, 张立华, 等. 操作条件对电氧化去除废水中有机污染物的影响研究[J]. 环境工程, 2017, 35(5): 6-10. [29] URANO Y, HIGUCHI T, HIROBE M. Substrate-dependent changes of the oxidative O-dealkylation mechanism of several chemical and biological oxidizing systems[J]. Journal of the Chemical Society Perkin Transactions, 1996, 6(6): 1169-1173. [30] 陈安妮, 童展梁, 姚佳超, 等. 电氧化处理印染行业膜后浓水的研究[J]. 工业水处理, 2021, 41(11): 51-55. [31] LUO C, MA J, JIANG J, et al. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2, UV/HSO5− and UV/S2O82−[J]. Water Research, 2015, 80: 99-108. doi: 10.1016/j.watres.2015.05.019 [32] FANG G-D, DIONYSIOU D D, AL-ABED S R, et al. Superoxide radical driving the activation of persulfate by magnetite nanoparticles: Implications for the degradation of PCBs[J]. Applied Catalysis B:Environmental, 2013, 129: 325-332. doi: 10.1016/j.apcatb.2012.09.042 [33] 李靖宇, 王宪森, 王晓林, 等. 载铁活性炭颗粒电极降解氯霉素污染废水性能研究[J]. 江西化工, 2021, 37(5): 56-58. [34] CHEN J, XIA Y, DAI Q. Electrochemical degradation of chloramphenicol with a novel Al doped PbO2 electrode: Performance, kinetics and degradation mechanism[J]. Electrochimica Acta, 2015, 165: 277-287. doi: 10.1016/j.electacta.2015.02.029 [35] XU J, LIU Y, LI D, et al. Insights into the electrooxidation of florfenicol by a highly active La-doped Ti4O7 anode[J]. Separation and Purification Technology, 2022, 291: 120904. doi: 10.1016/j.seppur.2022.120904 [36] 滕刚刚. PbO2-ZrO2复合电极电催化氧化降解典型PPCPs类污染物的研究[D]. 天津: 河北工业大学, 2020. -



 下载:
下载: