-
一般认为COD低于200 mg·L−1,COD/TN低于3.5—4.5或BOD5/TN小于3—5的污水属于低碳氮比污水[1]。据统计,我国污水处理厂进水中BOD5/TN小于4的占比约70%,污水碳氮比总体均偏低,南方地区尤其严重[2]。目前污水脱氮主要利用微生物反硝化技术,但对于低碳氮比污水,即使采用前置反硝化工艺如A/O、A2O等,仍不能满足总氮(TN)脱除一级A标准,这通常是由于二级出水中硝酸盐氮含量过高,需要再进行深度脱氮处理。为实现低碳氮比污水的达标排放,目前主要通过在缺氧反硝化阶段外加有机碳源,但这增加了污水处理成本与二次污染风险。为此,不依赖有机碳源的硫自养反硝化技术广受关注,成为解决低碳氮比污水无法达标脱氮问题的首选。
硫自养反硝化技术是利用H2S、S、
$\mathrm{S}_{2} \mathrm{O}_{3}^{2-} $ 、$ \mathrm{S}_{4} \mathrm{O}_{6}^{2-}$ 、$\mathrm{S} \mathrm{O}_{3}^{2-} $ 等为电子供体,CO2、$\mathrm{HCO}_{3}^{-} $ 、$\mathrm{CO}_{3}^{2-} $ 作为碳源[3],将硝酸盐还原为氮气的技术,具有无需外加有机碳源和污泥产量少两个重要优势[4]。对于低碳氮比污水深度脱氮效果显著,且经济性较高,常见于固定填充床反硝化滤池[5]。以单质硫为电子供体时,随着反应进行[6](式1),持续产生的酸会抑制微生物活性,必须添加碱缓冲材料来维持pH稳定在适宜范围内。
碱度材料有碳酸钙、碳酸氢钠以及碳酸亚铁等[3,7],但在实际大规模工程应用中,常常由于硫磺电子供体材料与碱缓冲材料密度不同,两者相互分离,导致微生物与这些填料在反应器内较难实现均匀传质,进而使得反硝化效果与酸碱度缓冲性能均降低。为解决上述问题,在我们之前的研究中开发了能同时充当电子供体与碱缓冲材料的碳-硫复合材料[6],并证实了其高效处理低碳氮比硝酸盐废水的可行性。但目前缺乏针对复合材料深度脱氮技术的工程应用参数如投加量、粒径、进水硝酸盐氮负荷等对脱氮效果影响的研究,难以精准指导实际工程应用。此外,由于微生物代谢反应的本质为酶促反应,因此体系动力学参数如传质系数、最大反硝化速率和Michaelis-Menten常数等对于深入理解微生物代谢过程介导的污染物去除机理至关重要[8],然而目前尚未对反应的动力学行为进行研究。
基于上述问题,本研究采用模拟低碳氮比硝酸盐污水为实验进水,构建了复合硫自养反硝化体系,探究硫-碳酸钙型复合材料的工程应用参数如投加量、粒径对体系反硝化性能的影响,以及体系抵抗进水硝酸盐氮浓度冲击的能力,同时考察反硝化过程中硝酸盐变化的动力学行为。
-
复合材料制备方法参考梁晶等[6]的研究,根据反应式(1)优化出的最佳S/C为1.72∶1,主要利用了单质硫与碱度材料在熔点上的显著差异(S0−112.8 ℃和CaCO3−825 ℃,标况下)。具体为,将元素硫粉在115—125 ℃间加热熔化后,缓慢均匀地将石灰石粉末铺撒在液态硫表面,用药匙均匀搅拌,在空气中冷却后,破碎和筛选获得的合成颗粒即为碳−硫复合材料,筛分出粒径为2.50—4.75、1.00—2.50、0.45—1.00、0.125—0.45 mm的4组,置于密封袋中紫外灭菌2 h备用。
实验原水为模拟低碳氮比污水,配水使用的化学药剂为:葡萄糖,磷酸二氢钾,硝酸钠,均为AR级。3种配水中硝酸盐氮浓度分别为15、30、50 mg·L−1,COD均为100 mg·L−1,TP均为1.5 mg·L−1,C/N比依次为6.7、3.3与2.0,亚硝酸盐氮与氨氮均未检出。
原活性污泥取自清华大学污水处理站二沉池,实验使用的接种污泥为经过3个月培养驯化后的。
-
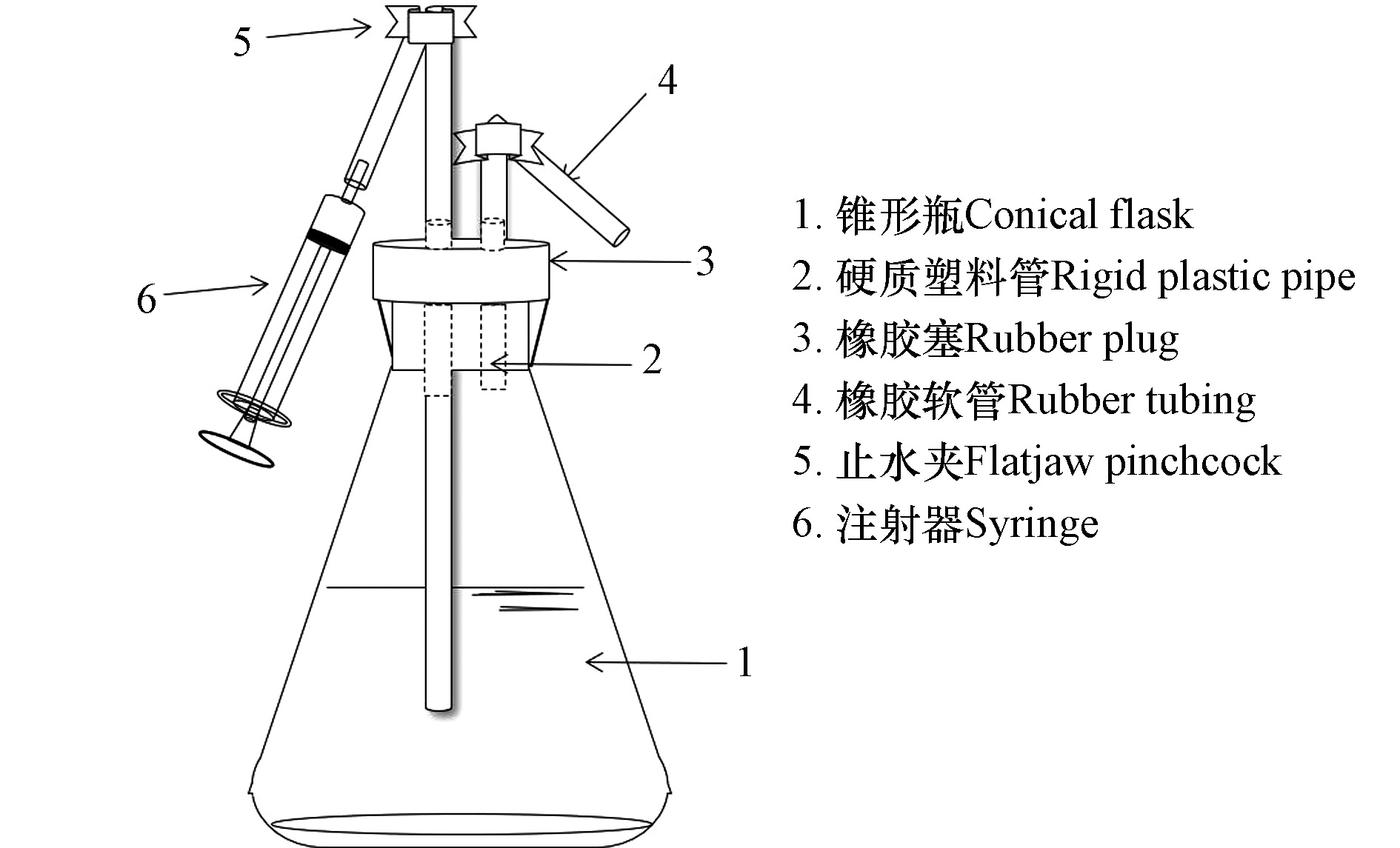
本研究实验装置如图1,包括复合材料−微生物反硝化反应器和注射器。注射器用于实验水样采集,反硝化反应器由带有密封橡胶塞的锥形瓶(500 mL)、硬质塑料管、橡胶软管与止水夹构成。每个反应器内分别添加对应不同浓度模拟硝酸盐污水、复合材料与活性污泥。
-
反应器内加入500 mL模拟废水,120 ℃下高压蒸气灭菌30 min。冷却后加入复合材料和5 mL活性污泥,通过胶管用氮气对混合液吹脱5 min,止水夹密封,然后置于温度为(25.0 ± 0.5) ℃,转速150 r·min−1的恒温震荡培养箱中,实验周期15 d。
对照组1仅加入活性污泥,对照组2仅加入复合材料,进水
$\mathrm{NO}_{3}^{-} $ -N浓度均为30 mg·L−1,复合材料投加量均为5 g·L−1,粒径均为1.00—2.50 mm。不同投加量实验设置:进水
$ \mathrm{NO}_{3}^{-}$ -N浓度为30 mg·L−1,复合材料粒径为1.00—2.50 mm,投加量分别为1、5、10 g·L−1。不同粒径实验设置:进水
$\mathrm{NO}_{3}^{-} $ -N浓度为30 mg·L−1,复合材料投加量为5 g·L−1,粒径分别为2.50—4.75、1.00—2.50、0.45—1.00、0.125—0.45 mm。不同进水
$\mathrm{NO}_{3}^{-} $ -N浓度实验设置:复合材料投加量为5 g·L−1,粒径1.00—2.50 mm,$\mathrm{NO}_{3}^{-} $ -N浓度分别为15、30、50 mg·L−1。 -
水质与污泥检测方法均参照《水和废水监测方法第四版(2002)》,
$\mathrm{NO}_{3}^{-} $ -N采用紫外分光光度法,$\mathrm{NO}_{2}^{-} $ -N采用N−(1−萘基)−乙二胺分光光度法,NH4+-N采用纳氏试剂分光光度法,pH采用玻璃电极法,MLSS采用重量法。实验中每组取两个平行样,实验结果由平均值与标准偏差表示。以硝酸盐为单一底物时,假设实验中微生物处于平衡生长稳定期,在降解与生长过程中无毒性物质存在,可用Michaelis−Menten方程描述反硝化动力学过程[9],进一步比较分析脱氮效果。
其中,Km为米氏常数,其值等于反应速率为最大反应速率一半时的底物浓度,Km值越小,意味着酶与底物亲和力越强;Vmax表示最大反应速率;Ci表示硝酸盐底物浓度。
利用Lineweaver−Burk法作图,对其进行线性回归拟合可得常数Km和Vmax,如式3。
当Ci>>Km时,符合零级动力学,Ci<<Km时,反应更接近一级动力学。本研究通过零级、一级与Michaelis−Menten方程动力学进行拟合分析。
IBM SPSS Statistics 20软件(IBM,Chicago,USA)进行主成分分析(PCA)和层次聚类分析(HCA)。不同投加量下,对不同反应时间的
$\mathrm{NO}_{3}^{-} $ -N、$\mathrm{NO}_{2}^{-} $ -N和$ \mathrm{NO}_{4}^{+}$ -N浓度构建成分矩阵。进行KMO和Bartlett球形检验后,对成分矩阵进行线性变换,基于特征值确定主成分数。主成分分析将反应周期中离散天数降维,确定反应周期中各单元子周期间的差异性,支撑更精确的模型拟合。将反应周期中离散天数的污染物变化数据通过离差平方和法(Ward’s method)进行层次聚类分析,该方法使用欧几里德距离作为相似性度量[10]。 -
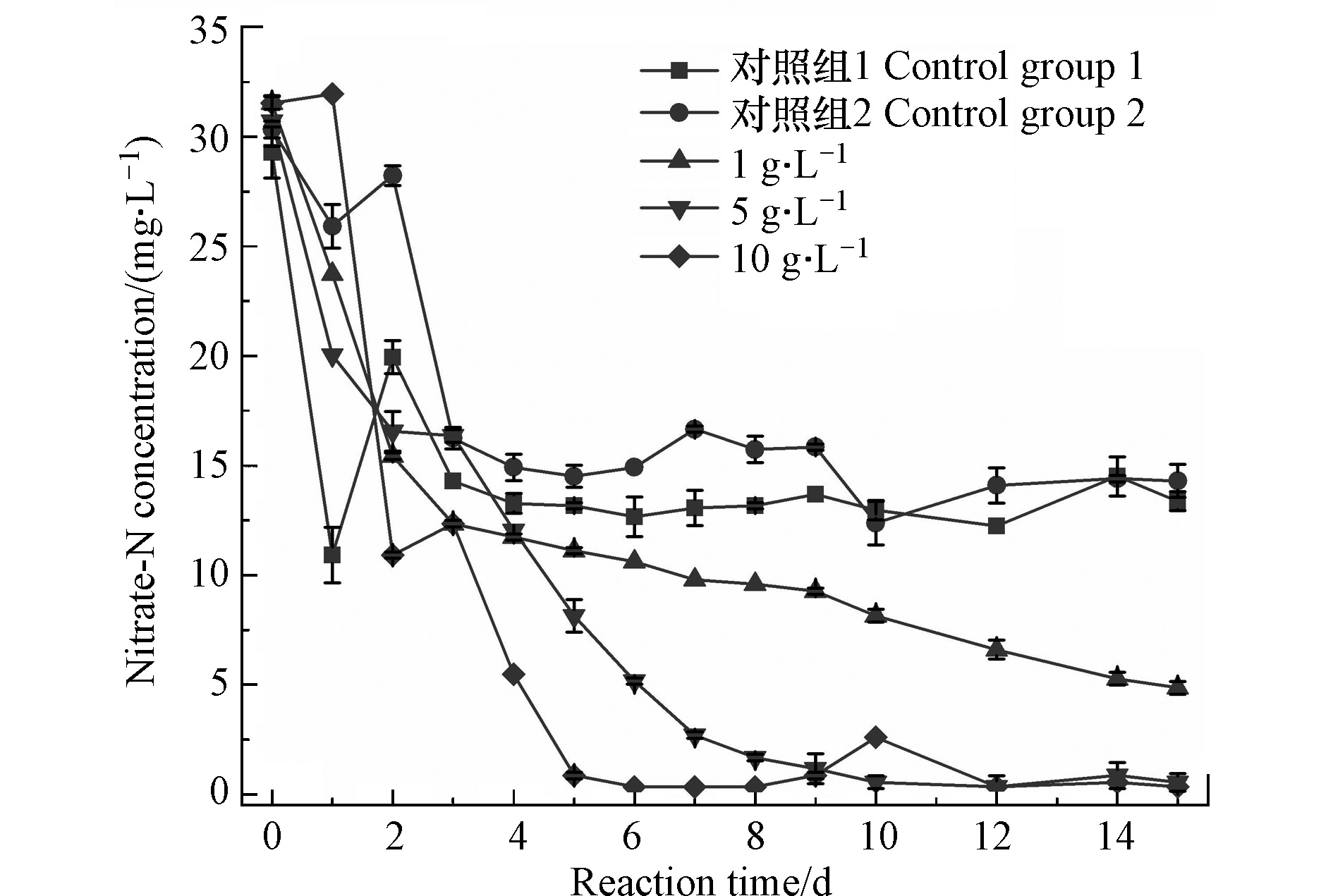
如图2所示,两对照组
$\mathrm{NO}_{3}^{-} $ -N浓度均在实验第3 d后不再下降,而实验组在第3 d后能继续下降,体系内复合材料与自养活性污泥是完成反硝化的必要条件。体系0—3 d出现微生物适应期的硝酸盐波动,但反硝化速率相近,第3 d后开始出现明显差异。对不同反应天数硝酸盐去除行为的有关数据($\mathrm{NO}_{3}^{-} $ -N、$\mathrm{NO}_{2}^{-} $ -N、$\mathrm{NH}_{4}^{+} $ -N和pH值)进行主成分分析,基于反应天数的显著差异性进行数据降维,结果如图3所示,从统计学角度证明体系存在至少两阶段反应。参考对照组变化,将实验组分为两个阶段的反硝化反应:0—3 d异养反硝化为主,3—15 d自养反硝化为主。这是由于硫自养−异养混合存在时,往往异养反硝化优先启动。李翔等[11]认为有机物作为电子供体的竞争性强于单质硫,仅当有机物浓度低时,自氧反硝化菌占据优势,这种现象在木屑−硫磺[12]、硫磺−甲醇、乙醇、醋酸钠[13],以及硫铁矿−葡萄糖[14]等自养异养共存体系中普遍存在。动力学拟合结果如图4所示,在0—3 d零级反应拟合效果较好(R2≥0.90),1、5、10 g·L−1投加量下反应速率常数分别为6.59、7.08、7.86 mg·L−1·d−1N,随着投加量增大而增大。第3天后,1 g·L−1投加量下仍用零级反应拟合效果最优(R2=0.99),但速率常数降低90%以上,为(0.63±0.02) mg·L−1·d−1N。微生物介导的酶促反应在底物浓度基本一致时,电子数量便成为重要影响因素[15]。1 g·L−1投加量下无机碳源与电子供体少,使得反应后期以自养反硝化为主时,微生物数量与生物膜相对较少,电子传递效率低,最大反硝化通量受到限制,表现为恒定速率的零级反硝化代谢反应,最大去除率仅85.2%。而5 g·L−1和10 g·L−1投加量下更符合一级动力学反应(R2≥0.93),说明缺少碳源时,投加5 g·L−1及以上复合材料能够满足微生物生长需求,生物量较大,最大反硝化通量受到硝酸盐底物浓度限制。其中,5 g·L−1与10 g·L−1投加量下,均可达到99%以上的去除率,但一级反应速率常数分别为0.40 d−1和0.97 d−1,随着电子供体数目增加,反应速率增大。
Michaelis−Menten方程动力学拟合参数结果如表1,投加量增大Km逐渐降低,Vmax上升。相同底物浓度下,复合材料投加量对于酶促反应参数存在较大影响。这意味着不同投加量下,电子供体数量能够影响微生物相关行为,充足的电子供体使得酶与底物亲和力增强,促进了反硝化进行。
硫是无极性矿物,几乎不溶于水,其从固相转化到生物膜表面的传质效率是影响反硝化的重要因素,而粒径能够改变材料表面积进而影响传质效率[15]。不同粒径条件下,仅粒径范围在2.50—4.75 mm间实验组中硝酸盐最大去除率为52.7%,其余粒径分布在0.125—2.50 mm的3组,硝酸盐去除率均在91%以上,最高可达99.9%。
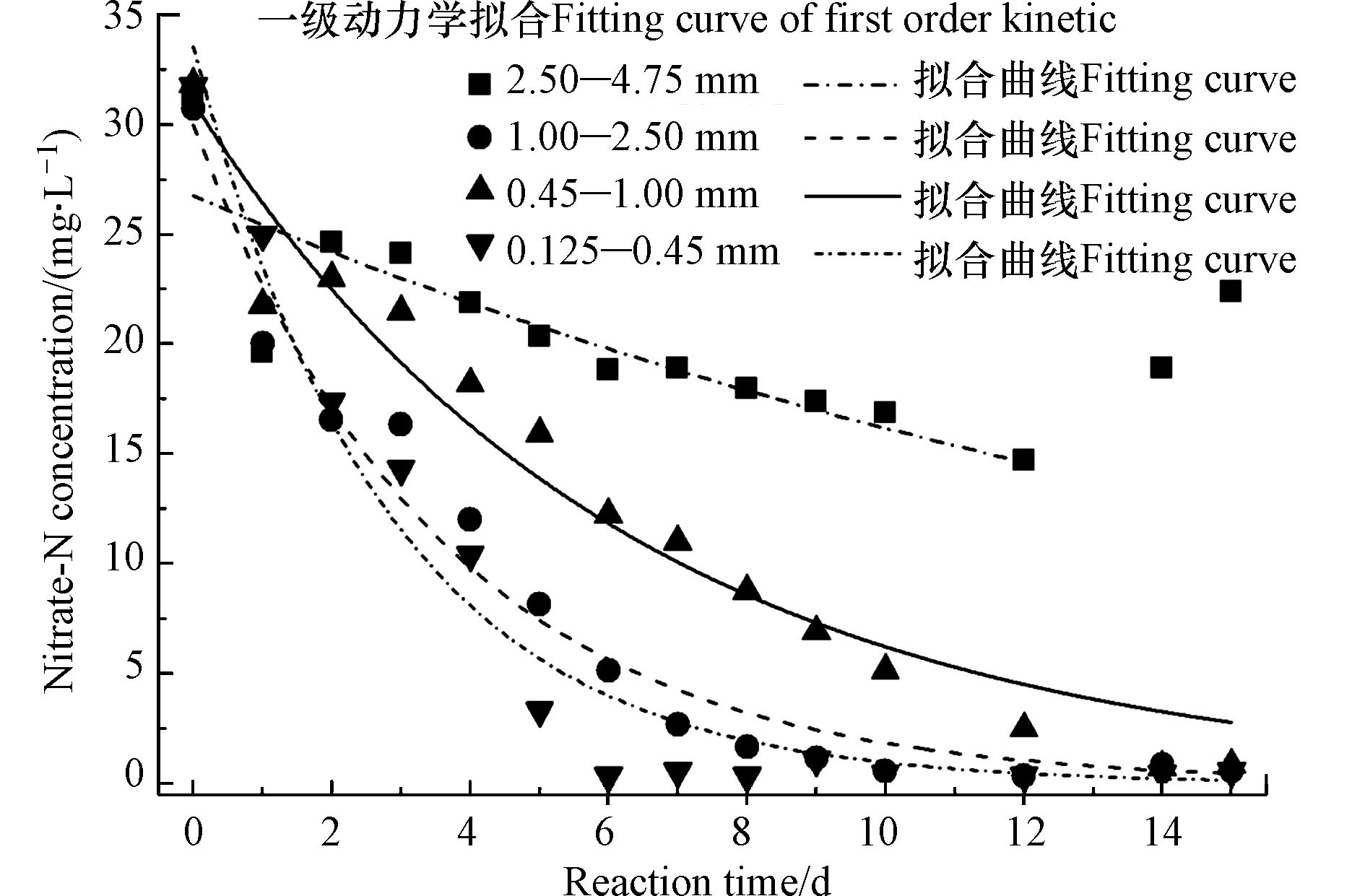
复合材料在不同粒径下反硝化动力学拟合如图5所示,实验周期结束,仅粒径在2.50—4.75 mm范围时存在硝酸盐积累,无法高效去除硝酸盐。这可能有两方面原因,一是粒径大时材料与生物膜接触表面积相对较小,且密度大易沉降至反应器底部,使溶液内硝酸盐向固相复合材料的扩散传质效率降低[16];二是随着活性污泥不断团聚、吸附在复合材料表面,使得表面活性位点逐渐饱和,且内部传质的孔道被表面代谢物质堵塞,内部硫元素无法被生物膜有效利用[17]。该粒径下反应器后期硝酸盐浓度上升是由于操作取样器取样时有空气流入,发生了硝化反应,这在反应器内亚硝酸盐含量下降及pH略微下降上得到了印证。
在投加量为5 g·L−1时,不同粒径下体系也存在两个阶段的反应,但一级动力学拟合效果也较好,故不再进行两个阶段的讨论。拟合常数如表2,目前一般认为随着粒径减小反硝化速率增大,有研究表明硫磺颗粒大小在2.8—16 mm之间时,颗粒越小反硝化速率越高[18],但本研究中复合材料粒径减小到0.45—1.00 mm时,反硝化速率没有对应增加,这种现象主要考虑到两方面,一是存在实验误差,二是微生物活性可能受到复合材料内部缺氧区大小的影响。陈浬等[19]利用乙酸钠为补充碳源进行尾水深度脱氮,发现在C/N比小于4时,作为生物载体的石英砂填料粒径越小,硝酸盐去除率越高。但安鹏等[17]在利用好氧颗粒污泥去除总氮(TN)时发现,粒径在0.5—1.0 mm时,颗粒内部形成的缺氧区较小,而反硝化菌多为兼性厌氧菌,不利于微生物高活性利用;当粒径大于1.5 mm时,虽然内部缺氧区大,但水力停留时间过长,内部孔道会被代谢物质堵塞,只有粒径为1.0—1.5 mm时具有最高的硝酸盐与总氮去除速率。这与本实验的结果相似,意味着硫−碳酸钙复合材料内部可能与活性污泥颗粒类似,同样含有缺氧区,且缺氧区大小能影响反硝化菌的活性。Kostrytsia等[20]对硫磺材料内部进行SEM扫描,发现硫磺内部呈现孔洞与孔道形状,且附着有活性污泥,说明硫磺材料内部具有形成缺氧区的传质孔道。本实验中使用的硫-碳酸钙复合材料在使用前后的内部结构有待进一步探究。
如表3,随着粒径减小,Km与Vmax均下降。Km减小意味着酶与底物的亲和力不断增大,但Vmax反而下降,出现这种现象的可能原因考虑是:酶与底物在反应过程中有生成中间复合物的稳态阶段,当结合过于紧密时,产物就难以分离,故而最大酶促反应速率下降。米氏常数的这种变化规律在其他实验体系中也常有出现,如朱睿、初志战等[21-22]对木瓜蛋白酶活性影响因素的研究中,发现有机溶剂浓度与种类对酶活性的影响可分为两种情况,其一随着溶剂溶度升高,酶与底物结合力下降,但最大反应速率上升;其二随着溶剂浓度升高,酶与底物结合力升高,但最大反应速率下降。此外,在董晓英等[23]对白菜吸收积累硝酸盐的研究中,报道了较高的Vmax与较高的硝酸盐吸收能力具有相关性,而不受Km值的大小影响。
硫磺材料粒径减小时,宏观反硝化速率应该增大[18],但最大酶促反应速率一直减小,这表明在该反应过程中可能存在其他影响反应速率的因素,如电子供体转移到生物膜内部的传质速率,粒径小时具有较大的比表面积,传质速率快。当达到极限酶催化反应速率后(即Vmax),宏观反应速率的提升可能主要依赖于传质过程。
进水NO3--N浓度不同时,3组反应器内NO3--N去除率均达95%以上,对其进行动力学拟合发现均符合一级动力学反应(R2>0.94)。如图6所示,随着进水
$\mathrm{NO}_{3}^{-} $ -N浓度增大,反硝化速率常数分别为0.24、0.28、0.31 d−1依次增大,这与已有研究中依靠硫进行自养反硝化的一级拟合常数相近[6,12]。丁清华等[24]发现硝酸盐浓度低于200 mg·L−1时反硝化速率与进水硝酸盐浓度正相关,本实验中该相关性不显著(P>0.05)。底物浓度成为影响Km与Vmax的主要因素时,随着硝酸盐浓度提高Km依次增大,在50 mg·L−1增幅最大,如表4所示。这可能是由于较多硝酸盐进入生物膜内与硝酸盐还原酶不紧密结合引起的亲和力下降,以利于迅速反应还原硝酸盐。但最大酶促反应速率同样在50 mg·L−1时增幅较高,在Km明显增大时,Vmax加快上升,这可能与底物刺激下反硝化微生物数量增多或者产生的相关还原酶增多有关。为了探究复合材料深度去除硝酸盐氮的可行性并优化复合材料的使用参数,本研究采用模拟废水进行静态批实验,探究不同实验参数对构建的复合硫自养体系反硝化脱氮的影响,为日后进行连续流实验或用于实际污水中,在复合材料投加量与破碎程度上提供了参考;此外,对复合硫自养反硝化体系内硝酸盐去除的动力学行为研究也为连续流实验的进水浓度、水力停留时间等提供了参考。
-
目前报道的完整的反硝化去除硝酸盐途径为
$\mathrm{NO}_{3}^{-} $ →$\mathrm{NO}_{2}^{-} $ →NO→N2O→N2,分别由不同的还原酶(NAR/NAP、NIR、NOR、NOS)控制,各个阶段存在对电子供体的竞争[25]。如图7所示,对照组与实验组在1 d后均出现亚硝酸盐积累,但对照组在第3 d后亚硝酸基本无变化,而实验组持续仍在变化,进一步印证了对照组中反硝化作用停滞。实验组中在1 g·L−1投加量下亚硝酸盐浓度持续上升,而5 g·L−1与10 g·L−1投加量下亚硝酸盐浓度分别增加至10.8 mg·L−1与5.8 mg·L−1左右后开始下降。亚硝酸盐的产生本质上是由于微生物利用硝酸盐进行呼吸作用后形成的产物。对比亚硝酸盐开始下降的时间发现,两者均在硝酸盐浓度到(5.33±0.22)mg·L−1时开始下降,这表明基于该复合材料的自养反硝化体系内,硝酸盐可能抑制了亚硝酸盐还原酶(NIR)的合成或活性,且抑制浓度在5 mg·L−1左右,这是由于反硝化电子传递链中硝酸盐夺取电子能力强,使NIR接收不到电子而丧失活性,引起亚硝酸盐积累[20,26]。在进水硝酸盐浓度相同时,亚硝酸盐积累量与硝酸盐去除速率显著相关(P<0.05)。投加量增大时,硝酸盐去除速率依次加快,亚硝酸盐积累量逐渐减少。如图7所示,随着粒径减小,亚硝酸积累量没有相应降低,粒径为1.00—2.50 mm与0.45—1.00 mm时,亚硝酸盐积累量最高分别为(10.43±0.03) mg·L−1与(14.26±0.01) mg·L−1,这可能与粒径为0.45—1.00 mm的体系内硝酸盐浓度一直较高,抑制着亚硝酸盐的还原有关。
粒径0.125—0.45 mm、投加量为5 g·L−1与粒径1.00—2.50 mm、投加量为10 g·L−1的两组硝酸盐去除速率相近,但5 g·L−1投加量的亚硝酸盐积累量为(9.34±0.05) mg·L−1明显高于10 g·L−1投加量下亚硝酸盐积累量。这表明粒径能改变反硝化速率,投加量会影响亚硝酸盐积累量。亚硝酸盐积累量很大程度上与微生物能够利用的电子供体数量相关,电子供体数目多时,NAR与NIR的竞争程度低,硝酸盐还原的同时有部分亚硝酸盐能够得到电子被还原[27]。
如图7所示,不同进水硝酸盐浓度下亚硝酸盐最高积累量分别为(2.94±0.01)、(10.43±0.02)、(20.61±0.05) mg·L−1,亚硝酸盐积累率分别为19.6%、34.7%和41.2%,随进水硝酸盐浓度增大而增大,同样最早解除硝酸盐浓度抑制(5 mg·L−1),亚硝酸盐最先被还原。
-
所有反应器在实验周期内氨氮呈现上升下降不断波动的趋势。氨氮的产生与硝酸盐还原的另一个途径
$\mathrm{NO}_{3}^{-} $ →$\mathrm{NO}_{2}^{-} $ →$\mathrm{NH}_{4}^{+} $ ,即硝酸盐异化成氨(DNRA)作用有关,此时亚硝酸盐被还原为氨,反应过程如方程式4,该过程中的亚硝酸盐还原酶由nrfA基因编码[28]。DNRA作用的存在不利于硝酸盐污染水体完成脱氮,然而研究发现其在活性污泥或其他生物脱氮系统z中普遍存在[29],尤其在缺少氮源的条件下。但体系内氨氮不会一直升高,这是由于其可作为硫自养反硝化过程中微生物生长的氮源被利用[12],利于反硝化菌的细胞合成。在本研究中实验组出水氨氮浓度均符合一级A标准。
未添加复合材料的对照组1前期发生厌氧发酵作用,产生的酸性物质使得pH降低,之后发酵作用消失,异养反硝化进行,使得pH回升至中性。添加复合材料的对照组2体系中,pH最高为7.95,但之后稳定在7.45左右;不同投加量的实验组中,从1 g·L−1增加到10 g·L−1时,投加量越大,反应器体系中pH越高,这种现象说明增加复合材料投加量时,体系内溶解的碱度浓度会随着增大;而不同粒径的实验组中,随着粒径减小反应器体系中pH升高,这意味着反应器内复合材料质量相同时,由于粒径减少会使得比表面积增大,同一时刻溶解的碱度浓度会相对增大,故pH值略微升高,本研究整个实验周期所有反应器中pH范围在6.5—7.89。常见的自养反硝化菌最适生长和反硝化的pH多为7.0—8.0[5],其中Thiobacillus denitrificans约为6.90,可见复合材料对于提供稳定适宜的反硝化pH有重要作用。
-
(1)复合硫自养反硝化体系(CSAD)中,复合材料粒径为1—2.50 mm,与污水中硝酸盐氮比例为1/6(g/mg)时为最佳反硝化条件,平均硝酸盐去除速率为3.63 mg ·L−1·d−1N。
(2)在反硝化阶段,污水中
$\mathrm{NO}_{3}^{-} $ -N浓度降低为5 mg·L−1左右时,对$\mathrm{NO}_{2}^{-} $ -N的抑制作用减弱或消失。增大复合材料的投加量,能增大硝酸盐还原速率,且降低体系内$\mathrm{NO}_{2}^{-} $ -N积累量。(3)复合材料和自养反硝化菌共同支撑了反硝化过程,复合材料与污水中硝酸盐氮比例为1/30(g/mg
$\mathrm{NO}_{3}^{-} $ -N)时体系内电子供体数量不能满足微生物反硝化需求,引起反硝化速率突变;比例不低于1/6(g/mg$\mathrm{NO}_{3}^{-} $ -N)时基本不存在电子供体数量不足导致的反硝化速率突变,整个反硝化过程服从一级反应动力学。(4)复合材料反硝化过程米氏常数(Km)范围在1.1—40.2 mg·L−1间,最大反应速率(Vmax)在1.4—9.5 mg·L−1·d−1)间。增大复合材料投加量促进Vmax增大,减小复合材料粒径会引起Vmax下降。
复合自养反硝化材料处理低碳氮比硝酸盐污水
Research on the treatment of low C/N ratio nitrate sewage by composite autotrophic denitrification material
-
摘要: 作为一种典型的硝酸盐污水,低碳氮比硝酸盐污水的存在具有广泛性,且对生态环境和居民健康带来的危害日益突出。本研究构建了复合硫自养反硝化(CSAD)体系,基于硫-碳酸钙型复合材料与微生物,进行低碳氮比硝酸盐污水的深度处理,分析了材料投加量、粒径以及进水硝酸盐浓度对CSAD的影响,并探究了硝酸盐去除的动力学行为。结果表明,体系中NO3--N去除率为52.7%—99.9%,NO2--N在NO3--N浓度降低至5 mg·L−1左右时由积累转变为迅速下降;复合材料投加量较低(1 g·L−1)时,自养阶段体系内电子供体数量不能满足微生物反硝化需求;增大投加量可提高NO3--N去除速率并降低反应过程中NO2--N积累量。当粒径大于2.5 mm时,复合材料利用效率显著降低。Michaelis−Menten方程拟合表明,投加量增大提高了酶与底物亲和力,促进Vmax增大,粒径减小增大了亲和力,但Vmax却减小。进水NO3--N浓度增大至50 mg·L−1时,亲和力显著降低,但Vmax增大可能与微生物数量增多有关。本研究从动力学角度上揭示了不同工艺参数对复合材料反硝化过程的影响,同时也为基于复合自养反硝化材料的低碳氮比污水深度处理的实际工程应用提供参考。Abstract: As a typical nitrate sewage, the existence of low C/N nitrate sewage is widespread and the harm to ecological environment and residents' health is becoming increasingly prominent. In this study, we constructed a composite sulfur autotrophic denitrification (CSAD) system based on sulfur-calcium carbonate composite material and microorganism for the advanced treatment of nitrate sewage with low C/N. The effects of material dosage, particle size and influent nitrate concentration on CSAD were analyzed, and the kinetic behavior of nitrate removal was explored. The results showed that the NO3--N removal rate of CSAD was between 52.7% and 99.9%, and NO2--N changed from accumulation to rapid decrease when NO3--N concentration decreased to around 5 mg·L−1. When the dosage of composite material was low (1 g·L−1), the number of electron donors in the autotrophic system can not meet the demand of microbial denitrification. Increasing the dosage of composite materials can increase the NO3--N removal rate and reduce the NO2--N accumulation in the process. The utilization efficiency of the composite materials decreased significantly when the particle size was larger than 2.5 mm. The fitting of the Michaelis-Menten equation showed that the increase in dosage increased the affinity of the enzyme and the substrate, thus it promoted the increase of Vmax. The decrease of particle size enhanced the affinity, but the Vmax decreased. When the influent NO3--N concentration increased to 50 mg·L−1, the affinity decreased significantly. However, the increase of Vmax may be related to the increase in the number of microorganisms. This study revealed the influence of different composite materials parameters on the denitrification process from the perspective of dynamics, and also provided a reference for the practical engineering application of advanced treatment of low C/N sewage based on composite autotrophic denitrification materials.
-

-
表 1 不同投加量米氏方程参数
Table 1. Parameters of Michaelis‐Menten equation with different dosage
投加量/(g·L−1)
DosageKm/
(mg·L−1)Vmax/
(mg·L−1·d−1)初始硝酸盐浓度
C0/(mg·L−1)R2 1 40.2 4.6 30 0.37 5 9.1 6.8 30 0.96 10 6.7 8.2 30 0.89 表 2 不同粒径下反硝化动力学拟合常数
Table 2. Fitting constants of denitrification kinetics under different particle sizes
粒径 /mm
Particle sizeK1/d−1 R2 C0/(mg·L−1) 2.50—4.75 0.05 0.74 26.8 1.00—2.50 0.28 0.97 30.0 0.45—1.00 0.16 0.96 31.0 0.125—0.45 0.35 0.97 33.5 表 3 不同粒径米氏方程参数
Table 3. Parameters of Michaelis‐Menten equation with different particle sizes
粒径 /mm
Particle sizeKm/(mg·L−1) Vmax/ [mg·L−1·d−1)] C0/(mg·L−1) R2 2.50—4.75 — — — — 1.00—2.50 9.1 6.8 30 0.96 0.45—1.00 4.9 3.4 30 0.93 0.125—0.45 1.1 1.4 30 0.50 表 4 不同进水硝酸盐浓度米氏方程参数
Table 4. Parameters of Michaelis‐Menten equation of different influent nitrate concentration
C0 /(mg·L−1) Km/(mg·L−1) Vmax/[mg·L−1·d−1)] R2 15 8.8 7.4 0.74 30 9.8 6.8 0.96 50 21.5 9.5 0.95 -
[1] 侯红娟, 王洪洋, 周琪. 进水COD浓度及C/N值对脱氮效果的影响 [J]. 中国给水排水, 2005(12): 19-23. doi: 10.3321/j.issn:1000-4602.2005.12.005 HOU H J, WANG H Y, ZHOU Q. Effect of influent COD concentration and C/N ratio on denitrification [J]. China Water & Wastewater, 2005(12): 19-23(in Chinese). doi: 10.3321/j.issn:1000-4602.2005.12.005
[2] 韦启信, 郑兴灿. 影响污水生物脱氮能力的关键水质参数及空间分布特征研究 [J]. 给水排水, 2013, 39(9): 127-131. doi: 10.3969/j.issn.1002-8471.2013.09.030 WEI Q X, ZHENG X C. Study on the key wastewater quality parameters influencing wastewater biological denitrification ability and their spatial distribution characteristics [J]. Water & Wastewater Engineering, 2013, 39(9): 127-131(in Chinese). doi: 10.3969/j.issn.1002-8471.2013.09.030
[3] TIAN T, YU H Q. Denitrification with non-organic electron donor for treating low C/N ratio wastewaters [J]. Bioresource Technology, 2020, 299: 122686. doi: 10.1016/j.biortech.2019.122686 [4] 李文超, 石寒松, 王琦, 等. 硫自养反硝化技术在污废水处理中应用研究进展 [J]. 水处理技术, 2017, 43(8): 1-6. LI W C, SHI H S, WANG Q, et al. Application progress of sulfur-autotrophic denitrification technology in wastewater treatment [J]. Technology of Water Treatment, 2017, 43(8): 1-6(in Chinese).
[5] 姚鹏程, 袁怡, 龙震宇, 等. 单质硫自养反硝化研究现状及展望 [J]. 现代化工, 2018, 38(6): 28-32. YAO P C, YUAN Y, LONG Z Y, et al. Status and prospects of researches on autotrophic denitrification of elemental sulfur [J]. Modern Chemical Industry, 2018, 38(6): 28-32(in Chinese).
[6] LIANG J, CHEN N, TONG S, et al. Sulfur autotrophic denitrification (SAD) driven by homogeneous composite particles containing CaCO3−type kitchen waste for groundwater remediation [J]. Chemosphere, 2018, 212: 954-963. doi: 10.1016/j.chemosphere.2018.08.161 [7] ZHU T T, CHENG H Y, YANG L H, et al. Coupled sulfur and iron(Ⅱ) carbonate-driven autotrophic denitrification for significantly enhanced nitrate removal [J]. Environmental Science & Technology, 2019, 53(3): 1545-1554. [8] ANDERSEN M, KARI J, BORCH K, et al. Michaelis-Menten equation for degradation of insoluble substrate [J]. Mathematical Biosciences, 2018, 296: 93-97. doi: 10.1016/j.mbs.2017.11.011 [9] GHANE E, FAUSEY N R, BROWN L C. Modeling nitrate removal in a denitrification bed [J]. Water Research, 2015, 71: 294-305. doi: 10.1016/j.watres.2014.10.039 [10] HE X, XI B, WEI Z, et al. Spectroscopic characterization of water extractable organic matter during composting of municipal solid waste [J]. Chemosphere, 2011, 82(4): 541-548. doi: 10.1016/j.chemosphere.2010.10.057 [11] 李祥, 马航, 黄勇, 等. 异养与硫自养反硝化协同处理高硝氮废水特性研究 [J]. 环境科学, 2016, 37(7): 2646-2651. LI X, MA H, HUANG Y, et al. Characteristics of a combined heterotrophic and sulfur autotrophic denitrification technology for removal of high nitrate in water [J]. Environmental Science, 2016, 37(7): 2646-2651(in Chinese).
[12] LI R, FENG C P, HU W W, et al. Woodchip-sulfur based heterotrophic and autotrophic denitrification (WSHAD) process for nitrate contaminated water remediation [J]. Water Research, 2016, 89: 171-179. doi: 10.1016/j.watres.2015.11.044 [13] HU S H, WU Y G, ZHANG Y J, et al. Nitrate removal from groundwater by heterotrophic/autotrophic denitrification using easily degradable organics and nano-zero valent iron as co-electron donors [J]. Water Air and Soil Pollution, 2018, 229(3): 56. doi: 10.1007/s11270-018-3713-5 [14] 张稳. 基于硫铁矿自养反硝化同步去除二级出水中氮磷的研究[D]. 北京: 中国地质大学(北京), 2019. ZHANG W. Synchronous N and P removal by pyrite-based autotrophic denitrification from secondary effluent[D]. Beijing : China University of Geosciences (Beijing ), 2019(in Chinese).
[15] DI CAPUA F, PIROZZI F, LENS P N L, et al. Electron donors for autotrophic denitrification [J]. Chemical Engineering Journal, 2019, 362: 922-937. doi: 10.1016/j.cej.2019.01.069 [16] 李志华, 曾金锋, 李胜, 等. 颗粒粒径与数量对硝化与反硝化过程的影响 [J]. 环境科学, 2012, 33(3): 903-909. LI Z H, ZENG J F, LI S, et al. Effect of size and number of aerobic granules on nitrification and denitrification [J]. Environmental Science, 2012, 33(3): 903-909(in Chinese).
[17] 安鹏, 杨凤林, 张捍民, 等. 不同粒径好氧颗粒污泥的性质比较 [J]. 给水排水, 2007, 33(z1): 45-49. doi: 10.3969/j.issn.1002-8471.2007.z1.012 AN P, YANG F L, ZHANG H M, et al. The comparison of different size of aerobic granular sludge’s properties [J]. Water & Wastewater Engineering, 2007, 33(z1): 45-49(in Chinese). doi: 10.3969/j.issn.1002-8471.2007.z1.012
[18] 马航, 朱强, 朱亮, 等. 单质硫颗粒尺寸及反应器类型对硫自养反硝化反应器启动的影响 [J]. 环境科学, 2016, 37(6): 2235-2242. MA H, ZHU Q, ZHU L, et al. Effect of element sulfur particle size and type of the reactor on start-up of sulfur-based autotrophic denitrification reactor [J]. Environmental Science, 2016, 37(6): 2235-2242(in Chinese).
[19] 陈浬, 王海, 黄韬, 等. 反硝化滤池深度处理污水厂尾水的效能研究 [J]. 中国给水排水, 2017, 33(13): 99-103. CHEN L, WANG H, HUANG T, et al. Treatment efficiency of secondary effluent of WWTP by denitrification filter [J]. China Water &Wastewater, 2017, 33(13): 99-103(in Chinese).
[20] KOSTRYTSIA A, PAPIRIO S, FRUNZO L, et al. Elemental sulfur-based autotrophic denitrification and denitritation: microbially catalyzed sulfur hydrolysis and nitrogen conversions [J]. Journal of Environmental Management, 2018, 211: 313-322. [21] 朱睿, 刘艳霖. 有机溶剂中尼龙布固定化木瓜蛋白酶动力学性能研究 [J]. 化工设计通讯, 2020, 46(8): 131-134. doi: 10.3969/j.issn.1003-6490.2020.08.087 ZHU R, LIU Y L. Kinetic characteristics of immobilized papain from neilon cloth in organic solvents [J]. Chemical Engineering Design Communications, 2020, 46(8): 131-134(in Chinese). doi: 10.3969/j.issn.1003-6490.2020.08.087
[22] 初志战, 陈纪鹏, 刘小林, 等. 醇类有机溶剂对木瓜蛋白酶催化活性的影响机理 [J]. 食品与生物技术学报, 2014, 33(10): 1112-1115. CHU Z Z, CHEN J P, LIU X L, et al. Effect on catalytic activity and conformation of papain with four kinds of organic solvent [J]. Journal of Food Science and Biotechnology, 2014, 33(10): 1112-1115(in Chinese).
[23] 董晓英, 李式军, 沈仁芳. 白菜不同品种对硝酸盐吸收积累差异原因初探 [J]. 园艺学报, 2003, 30(4): 470-472. doi: 10.3321/j.issn:0513-353X.2003.04.025 DONG X Y, LI S J, SHEN R F. The nitrate uptake and accumulation of Pak-choi [J]. Acta Horticulturae Sinica, 2003, 30(4): 470-472(in Chinese). doi: 10.3321/j.issn:0513-353X.2003.04.025
[24] 丁清华, 常刘伟. 进水中硝酸盐浓度对砂柱脱氮的影响 [J]. 广州化工, 2014, 42(2): 69-70. doi: 10.3969/j.issn.1001-9677.2014.02.025 DING Q H, CHANG L W. Effect of influent nitrate concentration on denitrification in sand column [J]. Guangzhou Chemical Industry, 2014, 42(2): 69-70(in Chinese). doi: 10.3969/j.issn.1001-9677.2014.02.025
[25] PAN Y, NI B J, YUAN Z. Modeling electron competition among nitrogen oxides reduction and N2O accumulation in denitrification [J]. Environmental Science & Technology, 2013, 47(19): 11083-11091. [26] 张庆芳, 李美玉, 王晓辉, 等. 微生物亚硝酸盐还原酶的研究进展 [J]. 微生物学通报, 2019, 46(11): 3148-3157. ZHANG Q F, LI M Y, WANG X H, et al. Research progress of microbial nitrite reductase [J]. Microbiology China, 2019, 46(11): 3148-3157(in Chinese).
[27] 艾小凡, 王鹤立, 陈祥龙. 地下水硝酸盐污染生物修复中的亚硝态氮积累研究 [J]. 环境工程, 2014, 32(1): 33-36. AI X F, WANG H L, CHEN X L. Experiment research on the problem of nitrite accumulation in groundwater during biological denitrification. [J]. Water Pollution Control, 2014, 32(1): 33-36(in Chinese).
[28] KUYPERS M M, MARCHANT H K, BORAN K. The microbial nitrogen-cycling network[J]. Nature Reviews. Microbiology, 2018, 16(5): 263-276. [29] 张新艳, 彭党聪, 万琼, 等. 活性污泥中硝酸盐异化还原成铵(DNRA)过程及其影响因素 [J]. 环境保护前沿, 2018, 8(2): 95-105. doi: 10.12677/AEP.2018.82012 ZHANG X Y, PENG D C, WAN Q, et al. Dominant factors of dissimilatory nitrate reduction to ammonia (DNRA) in activated sludge system: A comment [J]. Advances in Environmental Protection, 2018, 8(2): 95-105(in Chinese). doi: 10.12677/AEP.2018.82012
-



 下载:
下载: